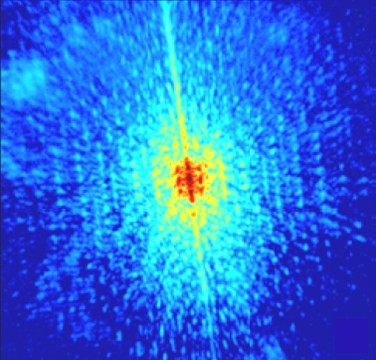
Emmanuelle Gouillart and a team at CEA Saclay, in collaboration with Jean-Luc Thiffeault at Imperial College, began by injecting a small amount of black dye into a cylindrical container of thick, transparent sugar syrup. The syrup was then mixed using a vertical rod moving in a figure-eight pattern while the concentration of the dye was measured using a digital camera. The degree of mixing was quantified in terms of the variation of the concentration of dye throughout the sample – with “grey” fluid being a completely mixed sample.
The experiment revealed that mixing occurred in the central region of the vessel, but the rate of mixing was limited by a very stubborn unmixed region near to the walls (see Mixing bowl ). This appeared to be caused by the syrup sticking to the walls and strips of this unmixed fluid were seen to move slowly from the edges into the centre, where they became mixed.
By studying time-sequences of the images and via numerical simulation, the team concluded that “laminar chaotic mixing” was occurring in the vessel. This is a well-known process by which fluids are mixed together gently without any turbulence.
However, the team was surprised at how slowly the mixing process was progressing. Instead of seeing a rapid exponential decay in the variation of dye concentration in the fluid that is normally expected in chaotic mixing, the team measured a much slower decay that obeyed a power-law. According to Thiffeault, this meant that the mixing was occurring hundreds of times slower than if the exponential decay was occurring.
The team was able to describe this power-law mixing in a theoretical model based on the “baker’s map” — the process by which a baker kneads dough by first stretching it and then folding it together. If this process is repeated, it leads to chaos. The effect of the sticky walls was modelled in terms of a periodic injection of strips, representing unmixed material, into the folds of the baker’s map.
While Thiffeault admits that engineers already have a good practical understanding of mixing, he says that industry could benefit from having a deeper knowledge of the physical processes involved. One field that could benefit from a better understanding of the effects of surfaces on chaotic mixing is microfluidics – which is concerned with the transport and mixing of fluids along very thin channels. The surface to volume ratio of microfluidics vessels is very large and therefore mixing is a great challenge to those designing such devices.
The sides of a vessel will always have some degree of stickiness, so Thiffeault believes that the best way to get rid of unmixed material at the edges is to rotate the vessel or use rod motions containing an extra “twist” to insulate the walls from the mixing region – something that is done in many kitchen mixers. The team are currently doing experiments on such stirring methods.