
Increasing global demand for renewable energy makes energy storage in batteries a critical area of research. Two-dimensional (2D) nanomaterials could be excellent candidates given their layered structures, offering well-defined pathways for ion movement. However, no single 2D material has yet demonstrated the perfect combination of properties needed. Writing in Nature Energy, Ekaterina Pomerantseva and Yury Gogotsi at Drexel University in the US propose a solution involving stacked nanomaterial architectures. The structures would combine the advantages of different 2D nanomaterials while eliminating their individual shortcomings.
Major technology breakthroughs within the past decade have produced a plethora of interesting nanomaterials. While these materials display a variety of useful properties, currently none can be used alone as an ideal battery electrode. Pomerantseva and Gogotsi propose the combination of multiple two-dimensional nanomaterials to achieve synergistic property enhancement. By interlayering different 2D materials within a deck-of-cards-like structure, the researchers believe higher energy and power densities can be achieved, as well as longer battery lifetimes.
Current battery limitations
The three critical battery properties are energy density, power density and lifetime.
Energy density is a measure of the amount of charge a material can store. An ideal phone battery, for example, would have a high enough energy density to allow the device to last a long time before dying. While some nanomaterials, such as transitional-metal dichalcogenides like molybdenum disulphide, show impressive energy densities, they also suffer from poor lifetimes, as the formation of a solid-electrolyte layer over time disrupts charge movement.
Power density refers to a battery’s ability to obtain charge. A phone battery with a high power density requires a short amount of time to charge, but also dispenses charge rapidly. Attention to application is required when considering a battery’s power density. The power density of nanomaterials can be manipulated by controlling particle morphology, which is not easily accomplished in a traditional 2D nanomaterial system.
The lifetime of a battery is typically limited by the reversibility of intercalation (the insertion of a molecule into a layered structure) and the mechanical strength of the battery material. Nanomaterial transition-metal oxides (e.g. V2O5) show appropriate intercalation but poor electron conductivity. Transition-metal carbides and nitrides (e.g. Ti2C and Ti2CN) display better conductivity and are mechanically robust but require a complex synthesis.
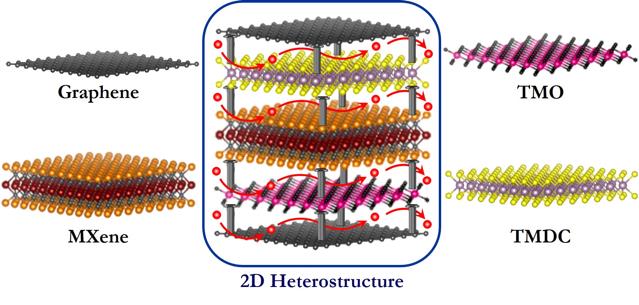
Deck-of-cards heterostructure
The heterostructures proposed by Pomerantseva and Gogotsi combine known 2D materials in a deck-of-cards arrangement, where each card has a different composition. The properties of the structure as a whole are better suited to energy-storage applications than those of the components taken individually.
For example, the interstratification of a transition-metal oxide and a transitional-metal carbide could produce a battery with exceptional ion intercalation, electron conductivity and mechanical strength. Different combinations of known 2D nanomaterials in the form of heterostructures could theoretically produce efficient batteries boasting high charge density, appropriate power density and long lifetimes. Selecting the appropriate elements to be placed between the layers in a heterostructure could not only further facilitate the movement of ions, but also improve the stability of the electrode.
Pomerantseva and Gogotsi stress the need for additional experiments to evaluate the energy-storage properties of such 2D heterostructures. They also highlight the importance of assembling “a genome for 2D material heterostructures for energy storage” to bridge the gap between the electronic materials and energy-storage communities. Completion of such an extensive project could “open a door to next-generation batteries with improved storage capabilities, faster charging and much longer lifetimes.”
Full details of the research are reported in Nature Energy.



