Ultrasound-mediated opening of the blood–brain barrier (BBB) for drug delivery can be successfully performed through a biocompatible plastic plate, a study on guinea pigs has demonstrated. The finding suggests that the cranial prosthesis could be used as a literal “window to the brain” for such therapeutic ultrasound techniques.
The BBB is the semipermeable interface between the brain’s capillaries and the cerebrospinal fluid. The barrier both protects the brain and maintains cerebral homeostasis by only allowing certain substances to pass across it. For example, lipid-soluble oxygen can diffuse directly across the barrier, while glucose is helped across by means of transporter proteins that bridge the endothelium. In contrast, other substances such as pathogens, blood solutes and assorted large molecules are kept out.
While this selective obstacle is ordinarily essential to brain health, it can provide an unwanted hurdle for the administration of drugs needed to treat a variety of neurological diseases. In fact, it has been estimated that the barrier can inhibit the passage of more than 98% of small-molecule drugs – and all large-molecule neurotherapeutics.
Various strategies have been proposed to sneak medicines past the border control that is the BBB. One approach, for example, involves packaging drugs with special molecules that trigger the endothelium’s transporter proteins, thereby tricking the barrier into actively admitting the therapeutic agents. Other solutions work instead by reversibly opening up holes in the barrier through which drugs can pass – the equivalent of prying open a hole in the border fence.
As their name suggests, microbubbles have diameters in the range of one-to-four microns. They are formed by encapsulating gas within a flexible lipid, polymer or protein shell. When injected into the bloodstream and targeted with low-intensity, pulsed ultrasound waves, they can act as “cavitation nuclei”, concentrating the incoming sonic energy on the BBB and temporarily opening up its intercellular junctions. This then allows large drug molecules to pass through to the brain’s functional tissues.
While clinical trials of this approach have proven highly promising, they are not without limitations. For one thing, it is impossible to visualize microbubble distribution prior to and during insonation, as the high impedance of the skull bone prevents transcranial imaging.
In their study, neurosurgeon Francesco Prada of the Fondazione IRCCS, Istituto Neurologico Carlo Besta in Milan and his colleagues propose that this limitation could be overcome in patients requiring a craniotomy by substituting the native bone that’s replaced at the end of the procedure with an acoustically transparent prosthesis.

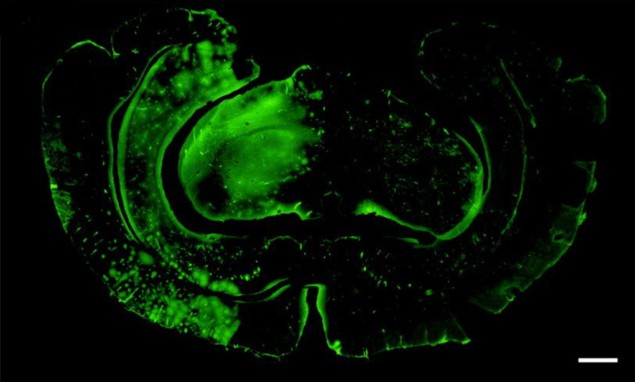
Working with 12 guinea pig brains that had been extracted, perfused and maintained in a water bath, the researchers experimented with insonating infused microbubbles in the model organs, both with and without the interposition of a 4 mm-thick plate of polyolefin – a biocompatible plastic material – between the transducer and the brain.
In contrast with many other BBB-opening experiments, the researchers used an unfocused planar transducer capable of insonating a much wider area of the brain than focused ultrasound approaches. They applied the ultrasound over one brain hemisphere after perfusion with microbubbles, monitoring the microbubble distribution with an ultrasound probe. The BBB opening was assessed by seeing whether FITC-albumin, a large fluorescent molecule, was capable of permeating the tissues of the brain.
“Our results showed that ultrasound-mediated blood–brain barrier opening is feasible across an acoustic transparent implantable cranial prosthesis,” Prada tells Physics World. “These findings, combined with previous work from our group showing that ultrasound imaging is feasible and safe across the prosthesis, are literally opening a window to the brain for ultrasound.”
Mapping microbubble distribution in the brain
Carmel Moran – an expert in translational ultrasound from the University of Edinburgh who was not involved in the present study – says that the team “convincingly confirmed” that the BBB could be opened when microbubbles were insonated through the polyolefin plate. She adds: “The results pave the way for future large animal and clinical trials utilizing this material as a long-term acoustic access point to the brain.”
With their initial study complete, the researchers are indeed now looking to move towards clinical trials. Alongside this, they say, they are also exploring whether the same setup might be used with other ultrasound treatments like sonodynamic therapy, in which sound waves are used to activate selectively cytotoxic drugs at specific locations in the body.
The study is described in Scientific Reports.



