The theme of the recent 2020 Joint AAPM|COMP Virtual Meeting was “Improving Health Quality. Increasing Global Impact”. The need to focus on global health is clear. While the burden of cardiac disease and cancer is rising disproportionately among low- and middle-income countries, such countries often have little or no access to quality imaging and radiotherapy technologies for timely diagnosis and effective treatment. There’s also a growing need for involvement of qualified medical physicists to ensure the safe delivery of high-quality care to patients.
“COVID-19 has taught us one lesson: global health is local health and local health is global health,” stated AAPM President M Saiful Huq. “Innovation in one part of the world, be it in the fight against COVID-19 or against cancer or any other disease, can benefit everyone across the globe. Collaborations between professionals from high-income and low- and middle-income countries can lead to innovations and development that can benefit all.”
In a dedicated symposium examining affordable medical physics technology for the developing world, speakers described a range of new and emerging low-cost healthcare systems.
Increasing imaging standards
Douglas Pfeiffer, a clinical medical physicist at Boulder Community Health, described a project to develop automated quality control (QC) for diagnostic and mammographic facilities.
“Implementation of regular QC programmes is poor for radiography throughout the world and for mammography, in many parts of the world,” he explained. “Regulations for quality assurance tend to be weak or non-existent.” And with diagnostic medical physics support minimal in many parts of the world, facilities often have little or no guidance on how to implement a quality assurance programme. “Imaging devices may go their entire lives without ever being tested,” Pfeiffer noted.
To remedy this situation, the IAEA set up a project to implement remote, automated and quantitative QC for radiography and mammography. The aim, explained Pfeiffer, is that one clinically qualified medical physicist will be able to support multiple facilities to ensure adequate and consistent imaging performance, which is important for areas of the world that are underserved by medical physicists.
In the proposed workflow, images of simple test objects are acquired locally and then either evaluated at the facility or transmitted to the medical physicist for remote analysis. The project team designed two inexpensive and easy to fabricate test objects – a radiographic phantom and a mammography phantom – both made from PMMA, copper and aluminium.

The team also developed an automated tool for image analysis (ATIA). The ATIA software extracts relevant data from the DICOM header of the image, and then reports a series of measured and calculated values: signal-to-noise ratio; signal difference-to-noise ratio; modulator transfer function; normalized noise power spectrum; and detectability index. The results are exported in a CSV file for further analysis.
Pfeiffer explained that the detectability index is a new metric that helps directly relate phantom measurements to clinical performance. It can also be directly linked to a specific clinical task, such as detection of microcalcifications or small nodules, for example.
“The IAEA programme allows a clinically qualified medical physicist and the local technologist to remotely monitor clinical performance with minimal investment and time per unit. Due to its simplicity, training is straightforward.” Pfeiffer concluded. “Regular radiographic QC has been neglected, even in developed countries. This system makes it possible to perform QC on a regular basis, since it takes just a single exposure per unit and analysis can be automated.”
Low-cost TB screening
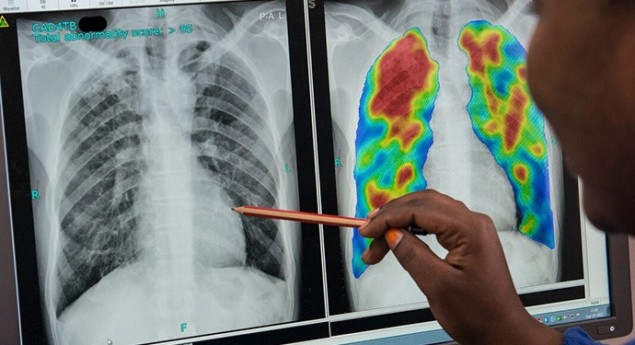
Relatively inexpensive imaging systems, such as X-ray and hand-held ultrasound scanners, combined with automated image analysis have enormous potential for countries with a lack of doctors. Bram van Ginneken from Radboud University Medical Center described two such systems – firstly a scheme to automate tuberculosis (TB) diagnosis. “Every day, about 4500 people die from tuberculosis,” he pointed out. “And the burden of disease is concentrated in Asia and Africa.”
In some aspects, van Ginneken said, TB has similarities to COVID-19. Both are diagnosed using RT-PCR (reverse transcription polymerase chain reaction) tests, which are expensive, time consuming and not always available. As such, for both infections, imaging also plays an important diagnostic role. One big difference, however, is that there’s an effective, $10 cure available for TB.
There is, therefore, a strong case for TB screening. And van Ginneken and colleagues are creating an automated screening process based on X-ray imaging. First, high-risk people receive a chest X-ray, which takes just one minute to record and process. If the X-ray looks suspicious, then the subject undergoes an Xpert diagnostic sputum test, waits two hours, and then receives a diagnosis. If TB is confirmed, they can be treated immediately.
Van Ginneken explained that the X-ray and Xpert machines can be easily set up, for example, on a bus that can travel between sites. He shared the example of a clinic in Zambia where the team placed their digital X-ray machine in a shipping container. He noted that even smaller systems are coming onto the market, such as a completely portable X-ray machine in a backpack. “It is quite heavy, but you can carry it anywhere,” he said. “There is an X-ray tube, a detector, a battery pack and a solar panel to charge the battery.”
To detect signs of TB on the X-ray images, the team developed computer-aided detection software called CAD4TB. The latest version of CAD4TB is based on deep learning, which can train classifiers to easily obtain very good performance. “It is already operational in over 45 countries throughout the world and we are screening about 5000 people per day,” said van Ginneken.
The team has also created a version of the software to detect COVID-19 on chest X-rays. “We have already received funding to test this CAD4COVID together with CAD4TB in a joint screening in Lesotho,” said van Ginneken. “The goal is to get people into the right part of healthcare system: find the severe COVID cases, less severe cases, TB cases and people who might have both diseases.”
Pregnancy risk prediction
Van Ginneken and colleagues are also applying deep learning techniques to help reduce maternal deaths by performing ultrasound imaging and analysis during pregnancy. “Every day, about 800 women dies as direct result of pregnancy,” he said. “99% of these deaths happen in low-resource countries.”
The scheme is based on a low-cost portable ultrasound device that can be directly attached to a smartphone for real-time image analysis. To analyse the ultrasound images and automatically detect any pregnancy risks, the team developed the BabyChecker software. This software uses deep learning to determine gestational age, predict delivery date, identify twins and detect a foetus in the breech position, which indicates a high-risk pregnancy.
While it is relatively simple to perform automatic measurements to determine gestation, it is essential to acquire the correct image frames. To achieve this without requiring extensive training, the team implemented a sweep protocol, in which the ultrasound probe is swept six times over the abdomen (three vertical, three horizontal). The resulting video contains all the required information.
“We have trained midwives in Ethiopia to perform these six sweeps, and saw that in two hours we can train somebody who has never used ultrasound before to perform sweeps in the right way,” said van Ginneken.
The deep learning software classifies every frame in the video, in real time, as one of six types: head, part-head, body, side view, detached transducer or other. These classifications can, for example, detect “head” frames located at the top of the sweeps, indicating a breech position, or find “head” frames at either end, suggesting the mother is having twins.
BabyChecker runs all of the deep learning networks in real time on the smartphone. The system also provides feedback during sweep acquisition to ensure the scans are performed correctly. “We found that countries with limited resources are very open to the use of AI to improve healthcare,” van Ginneken concluded. “With deep learning, we can rapidly develop these applications.”
Low‐cost arc therapy
Alongside medical imaging technologies, there’s also a real need to bring radiotherapy to lower-income countries. One approach, according to Magdalena Bazalova-Carter from the University of Victoria, could lie in the use of kilovoltage X-rays.

While kilovoltage X-rays are common in diagnostic imaging, they are not the obvious choice for radiotherapy. It is difficult to deliver a high dose to deep-seated targets at kilovoltage energies, while low machine output compared with megavoltage linacs necessitates long treatment times.
“But there are advantage of kilovoltage X-rays,” Bazalova-Carter explained. “The shielding for a kilovoltage beam doesn’t have to be seven feet of concrete and the technology used is not as expensive. You could save a lot of money by using kilovoltage X-rays, which might be interesting for low- and middle-income countries.”
With this in mind, Bazalova-Carter and her former PhD student Dylan Breitkreutz, in collaboration with Michael Weil, have developed a kilovoltage arc therapy (KVAT) system. The KVAT source uses one-dimensional scanning of the electron beams onto the treatment anode, combined with collimation, to generate a linear array of focused kilovoltage beams that can be used to deliver arc therapy to generate conformal spherical dose distributions at depth.
For treatment planning, the team combined Monte Carlo simulations using the EGSnrc code with the Radify treatment planning system developed at McGill University. As the KVAT collimators are designed to deliver spherical dose distributions, while human targets are typically not spherical, the team used sphere packing to deliver dose to irregular targets.
Bazalova-Carter showed an example of a lung cancer treatment plan. Comparing dose distributions of the KVAT lung plan with a stereotactic ablative radiotherapy (SABR) plan showed that a higher volume received lower doses with KVAT, as expected, but met all Radiation Therapy Oncology Group (RTOG) dose constraints. Dose–volume histograms revealed that dose to the ribs was higher with KVAT; however, the oesophagus dose, for example was higher for SABR. She noted that KVAT treatment time is indeed still an issue, taking about 49 min to deliver a 12 Gy fraction.
The KVAT system with a 200 kV beam is currently under development by industrial partner Precision RT.



