Tumour hypoxia, a condition in which oxygen concentration in tumour cells is significantly low, causes radioresistance and often occurs in non-small cell lung cancer (NSCLC). Swedish researchers have now developed a method to calculate an optimum radiation dose for hypoxic target volumes in NSCLC tumours, based on the use of PET images to assess tumour oxygenation. They examined a range of fractionation schedules for “dose painting” treatment plans and investigated the feasibility of using their method for calculating the dose required in hypoxic subvolumes (Med. Phys. 10.1002/mp.13514).
Hypoxia is heterogenous and dynamic in nature, and there are no generally accepted radiotherapy approaches for hypoxia mitigation. Stereotactic body radiotherapy (SBRT) is a recommended treatment for NSCLC because of its ability to deliver precisely targeted, high-dose irradiation in a small number of sessions. However, its short treatment duration does not allow enough time for the global reoxygenation that results from tumour shrinkage in longer treatments. Such treatments with extreme hypofractionation would be more effective if their impact on hypoxic tumours could be estimated, and coverage of hypoxic areas of tumours could be determined.

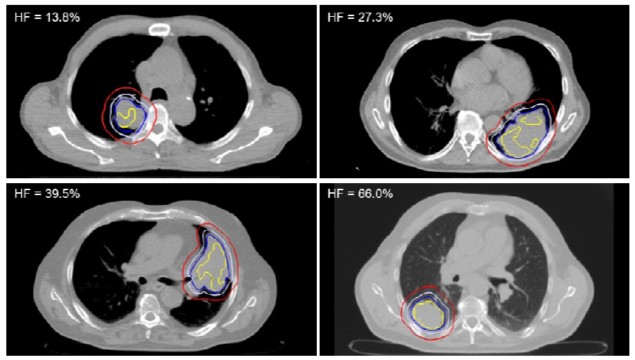
Led by Emely Kjellsson Lindblom from Stockholm University, the authors investigated the impact of imaging with 18F-flortanidazole (18F-HX4), a novel PET hypoxia radiotracer. The researchers applied a function that converts normalized tracer uptake to oxygen partial pressure (pO2) to the 18F-HX4 PET imaging data of 19 patients with NSCLC. They segmented hypoxic target volumes (HTV) based on a pO2 threshold of 10 mmHg, the upper limit of that conventionally considered the hypoxic threshold. The authors considered this to be the safest approach to ensure that all clinically relevant hypoxia would be included in the HPV.
For each patient, the researchers adjusted the gross tumour volume (GTV) to include the HTV when it extended beyond the GTV. They calculated the prescribed uniform dose required for 95% tumour control probability for the HTV, the target for hypoxia-based dose painting. They also determined the prescribed dose for the GTV excluding the HTV and the clinical target volume (CTV) excluding the GTV.
“By segmenting the tumour in this fashion, the uniform dose prescription can be performed for individual subvolumes, each with a more homogenous radiosensitivity than in the whole tumour volume,” the authors explained. They also performed calculations for fractionation schedules ranging from conventional radiotherapy to extremely hypofractionated SBRT for each tumour.
Eleven of the patients had an adjusted GTV less than or equal to 1% larger than the original GTV. In five patients, the adjusted GTV was between 1% and 5% larger than the originally delineated volume. The largest change of 74% was observed in the patient with the smallest GTV, 6.2 cm3, which was adjusted to 10.7 cm3 to encompass the HTV.
For all but one patient (who had a negligible HTV), the HTV dose was consistently higher than both the GTV minus HTV and CTV minus GVT doses. Interestingly, for patients in whom the HTV ρO2 distribution was more favourable, a lower dose was required despite a bigger volume. The researchers noted that treatment regimens with several fractions produced higher levels of tumour control probability than the single-fraction treatment regimen.
The doses calculated for these 19 patients were highly individualized, which the authors state makes a strong case for the need to segment tumours based on hypoxia PET imaging. “Because the method refers to the calculation of the doses to be prescribed, the time required to create the subsequent dose plan should not be affected, provided that the doses to be prescribed to the different volumes do not result in a technical challenge with respect to fulfilling the clinical goals,” Kjellsson Lindblom tells Physics World. She says that the research team is currently investigating the feasibility of creating clinically relevant dose plans following segmentation and dose prescription based on tumour oxygenation.
“Hypoxia dose painting based on segmentation alone may not may not be sufficient with respect to achieving local control,” the authors observed. “By converting the normalised tracer uptake to pO2, subvolumes can be delineated based on a hypoxic rather than an uptake threshold, and the dose that is required in order to overcome the resistance resulting from the pO2 distribution within that volume can be calculated.”
The next step will be clinical validation. “The predicted outcome according to the model needs to be compared with the actual outcome of treated patients,” explains Kjellsson Lindblom. “Using the pre-treatment hypoxia imaging data and dose plans for already treated patients, the method would be used to calculate the expected outcome for these patients, and by comparing these with clinical outcomes, our model could be validated or adjusted and improved according to the clinical findings.”



