One of the biggest challenges in cancer therapy is to develop drugs that are as selective as possible, so as to target cancer cells while leaving healthy surrounding tissues intact. Over the last decade, the development of chimeric antigen receptor (CAR) T cell-based immunotherapies has brought us significantly closer to solving this challenge. These therapies involve collecting from a patient’s blood the immune system T cells responsible for identifying and killing cancer cells, and engineering them to produce new surface proteins (CAR) that recognise specific markers – antigens – on the tumours. Once reinjected into the patient’s bloodstream, these CAR-T cells can identify and attack cancer cells more effectively.
While CAR-T cells have proved efficient for treating blood cancers such as leukaemia and lymphoma, solid tumours, such as found in the breast, liver or lung, have been more difficult to vanquish. Many of the markers characteristic to those tumours are also found in normal tissues, causing the destruction of both, as CAR-T cells do not distinguish between healthy and diseased cells. The challenge has hence shifted from “how do we target cancer cells” to “how do we do this while ensuring healthy tissues are left unharmed”.
A possible approach has recently been presented in two complementary articles by Wendell Lim’s research group at University of California San Francisco and Olga Troyanskaya’s group at Princeton and the Flatiron Institute of the Simons Foundation. The researchers combine machine learning and cell engineering techniques to create CAR-T cells that, instead of recognising just one antigen, use Boolean logic (AND, OR and NOT operators) to target combinations of up to three antigens. For example, if antigens A and B are mostly found in tumours but can also be present in healthy cells, while C is only found in normal tissues, the combination “A” OR “B” AND NOT “C” would help differentiate the tumour from normal tissues.
“Currently, most cancer treatments, including cell therapies, are told ‘block this’ or ‘kill this’,” explains Lim. “We want to increase the nuance and sophistication of the decisions that a therapeutic cell makes.”
Preventing off-target killing of healthy cells
In the first article, published in Cell Systems, the researchers investigated the efficiency of antigen combinations to distinguish normal and cancerous tissues in a database of the human genome containing 2358 antigens. A clustering-based score sorted over 2.5 million antigen pairs and approximately 60 million triple antigens. Pairing antigens using either AND or NOT logic gates significantly improved tumour recognition, outperforming well-established single antigens already investigated clinically, in 33 tumours and 34 normal samples.
These Boolean instructions can be programmed into CAR-T cells via synthetic Notch receptors (synNotch), one of the latest developments in cell engineering. Briefly, when a protein binds the Notch receptor, a portion of the receptor breaks off and heads for the cell nucleus, where it acts as a switch to turn on other genes. This allows cells to behave like molecular computers that can sense their environment and then integrate that information to make decisions.
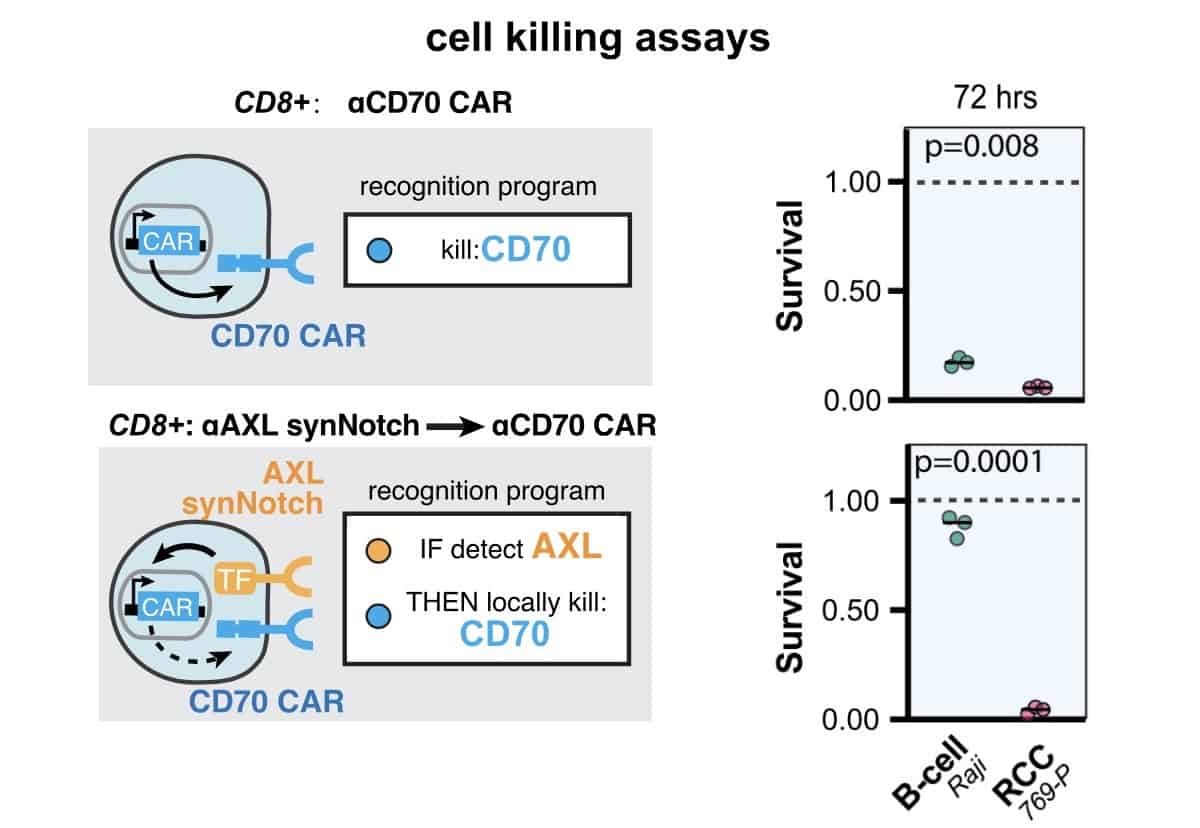
To prove the accuracy of the method, the researchers programmed synNotch receptors to recognise two markers found in kidney tumours, CD70 and AXL, using an AND gate. Targeted separately, CAR-T cells would result in off-target damage, as CD70 is also widely present in healthy blood cells and AXL can be found in healthy lung tissues. But targeting both using an AND gate not only suppressed their expression in tumours in vitro, it also ensured that normal tissue containing just one of these antigens were left unharmed. For example, Raji B cells, which are found in the blood and express CD70, had a survival rate close to 100% with the two antigens, while only around 20% survived when only CD70 was targeted.
Adding a third antigen in the combination helped improve the overall performance across several types of tumour. It also revealed the importance of NOT gates, with 92 of the top-100 combinations of gates for each cancer having at least one such gate. This further highlights the importance of NOT gates in preventing toxic cross reactions, while also significantly improving the correct identification of challenging tumours, such as cholangiocarcinoma, a type of cancer that forms in bile ducts.
New killing strategies
In a second study, published in Science, Lim’s research team expanded on their initial work and daisy-chained multiple synNotch receptors to create a host of complex cancer recognition circuits. The “plug-and-play” nature of synNotch enabled them to customize circuits with diverse Boolean functions, allowing for precise recognition of diseased cells and a range of responses when those cells are identified.
Such circuits can be used in complex scenarios. For example, an antigen localized on the surface of a cell can be targeted, and the decision whether or not to launch the killing process would then be tied to the presence of a second cancer antigen inside the cell. Since CAR-T cells are usually restricted to recognising extracellular antigens, which only represent about 25% of a cell proteome, resorting to this Boolean logic enables targeting of new cancer antigens. As the researchers demonstrated in vitro with melanoma cells, this dual intracellular–extracellular targeting approach both improved specificity and reduced off-target killing.

Blue light activates antibodies on demand
In vivo experiments also showed promising results. The researchers injected a mouse presenting different tumours in each flank – one with two antigens, one with the same two antigens plus an additional third – with a three-antigen-AND-gate T cell composed of three sequentially linked receptors. Allowed to autonomously explore and act on both tumours, the T cells rapidly cleared the three-antigen tumours while ignoring the two-antigen tumours on the opposing flank, similarly to the results observed in vitro.
The possibilities are endless as these smart cells can be designed to fight all kind of tumours. Lim’s group is now exploring how these circuits could be used in CAR-T cells to treat glioblastoma, an aggressive form of brain cancer that is nearly always fatal, using conventional therapies.
“You’re not just looking for one magic-bullet target. You’re trying to use all the data,” Lim says. “We need to comb through all of the available cancer data to find unambiguous combinatorial signatures of cancer. If we can do this, then it could launch the use of these smarter cells that really harness the computational sophistication of biology and have real impact on fighting cancer.”



