Researchers in Beijing have engineered the protein SDF-1α (stromal cell-derived factor-1α) to tether to collagen, enabling controlled release of SDF-1α to promote cell migration towards the site of injury in tendon (Biomaterials 162 22).
Tendon injury is common, and not only in athletes. Unfortunately, current treatments do not provide satisfying results due to a high tearing recurrence. To address this matter, many studies are investigating how to associate cells to a scaffold to promote regeneration. However, the administration of exogenous cells can lead to ethical issues and also raises the question of which cell source is the most suitable.
Another strategy is to attract endogenous cells to the scaffold using chemo-attractant molecules. SDF-1α induces the migration of different cell types, including mesenchymal stem cells, dermal fibroblasts and Achilles tendon fibroblasts. A limitation in the use of SDF-1α – despite the fact that it has been shown to trigger regeneration of several organs, including tendon – is that it diffuses too fast in vivo, which reduces its local concentration and thus its efficacy.
Jianwu Dai and his team at the Chinese Academy of Sciences have engineered SDF-1α by adding a protein fragment that can bind to collagen (collagen binding domain, CBD) to control its release. The authors chose collagen type I as the scaffold due to its good biocompatibility and mechanical properties. Also, type I collagen is the main component of the extracellular matrix (ECM), which is the scaffold that cells secrete to support themselves.
The researchers attached this CBD-SDF-1α to a type I collagen sponge and studied its behaviour in vitro and in vivo (in a rat Achilles tendon defect model). SDF-1α acts on cells through a receptor named CXCR4 localized on the cell surface. Tracking cells that express this marker provides a way to determine whether SDF-1α triggers their migration.
CBD-SDF-1α attachment and release
The authors first checked that the addition of the CBD to SDF-1α didn’t alter its bioactivity, and that it can attach to the collagen scaffold. They found that release of CBD-SDF-1α from the scaffold was slow. After nine days, 61% of the molecules were released, with more time required for complete release. This report showing a slow release profile of the molecule-of-interest is one of the few in the field.
Later, the researchers observed in vivo that CBD-SDF-1α scaffold triggers the recruitment of more CXCR4-positive cells than the controls of collagen sponges without SDF-1α and with native SDF-1α (not modified). These cells deposit ECM and tenascin C, a marker of the healing tendon. They also saw no excessive amount of cells from the immune system migrating to the injury area, suggesting that CBD-SDF-1α didn’t induce immune cell reaction.
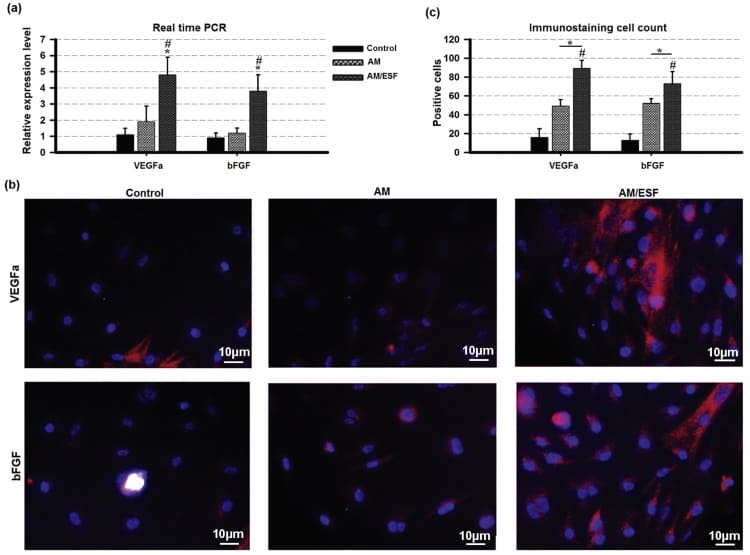
Formation of new tendon
The scaffold gave place to the formation of new tendon tissue after one week, although more time is required for it to resemble to a native tendon. The mechanical properties of the neo-tendon (the force that the tendon can withstand and the stiffness) were superior to those of the control systems. These superior properties are explained by the fact that, in vivo, tendon is made of collagen fibrils, which are highly organized and aligned. In this study, collagen fibrils in the CBD-SDF-1α group were larger and aligned, and the cells aligned with them.
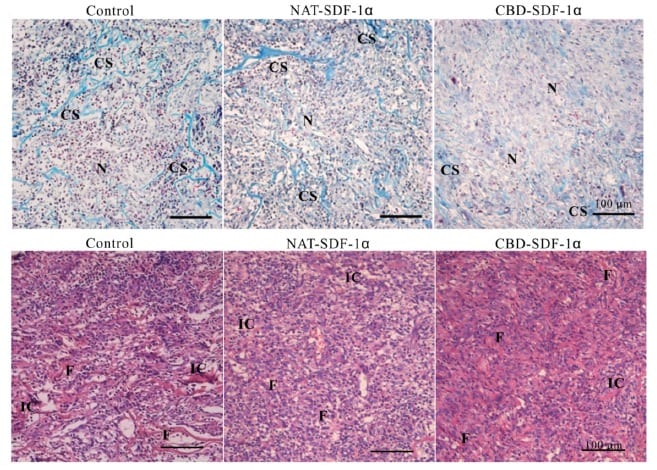
One limitation of most investigations is the formation of bone instead of tendon at the site of injury, mainly due to the differentiation of stem cells to bone cells. In contrast, in this work, CBD-SDF-1α scaffold didn’t promote bone formation.
This study is highly encouraging for the development of collagen sponges tailored with specific features for tissue engineering. In future work, this CBD-SDF-1α sponge should be studied in larger animal models and for other applications, such as healing bone, cartilage and many more.



