Two international teams of researchers have reached different conclusions about whether or not boron can form planar sheets similar to graphene. One team worked in the lab to identify hexagonal clusters of 36 boron atoms with a central hole. If joined together, the researchers believe that such structures could form a perfectly flat, atom-thick sheet that they have dubbed “borophene” – however, they have yet to see sheets. The second team used electronic-structure theory to calculate that such boron monolayers would spontaneously break down into bilayer structures. One of these bilayers would, the calculations suggest, have electronic properties similar to graphene, despite having a completely different atomic structure.
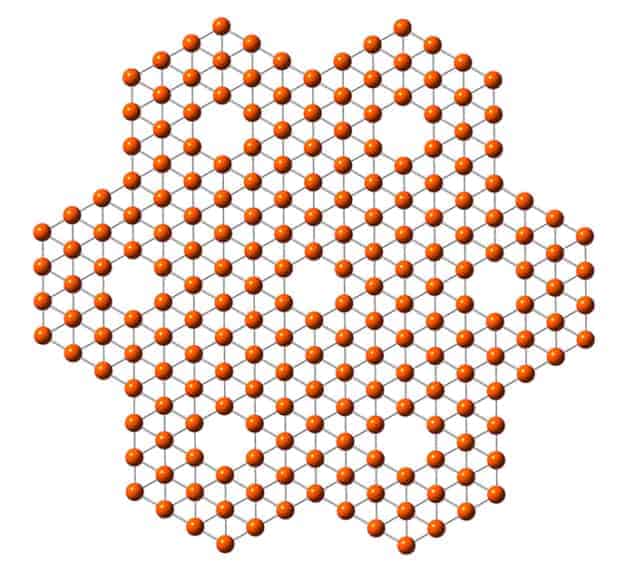
Graphene is a sheet of carbon just one atom thick that has attracted great interest because of its remarkable electronic and mechanical properties. Since graphene was first isolated in 2004, researchers have sought similar 2D materials, particularly those with different or complementary properties. One atom of interest is boron, which is the only non-metal in group three of the periodic table. Boron–boron bonds are not truly ionic, covalent or metallic. Instead, the bonds are both strong and highly delocalized. Like carbon, calculations suggest that boron should be able to form giant planar structures and fullerenes – structures such as buckyballs and nanotubes that have atom-thick skins. Indeed, boron nanotubes have been observed – although their precise structure remains uncertain. In 2007 Hui Tang and Sohrab Ismail-Beigi of Yale University predicted a stable planar boron structure containing regular hexagonal holes, which they called the α-sheet.
Frozen boron
Now, Lai-Sheng Wang and colleagues at Brown University in the US and Tsinghua University in China have tried to create graphene-like boron by using a laser to vaporize atoms from the surface of bulk boron. The vapour is then frozen rapidly with a jet of helium gas to form clusters of atoms. Mass spectrometry is used to separate out the negatively charged clusters and identify their masses, and therefore how many atoms they contain. Finally, the tiny particles are analysed using photoelectron spectroscopy, which measures how much energy is needed to liberate an electron from a nanoparticle.
The team focused on nanoparticles containing 36 atoms (B36–) and found that the electrons were strongly bound, implying that the clusters were very stable. To find out their structure, the researchers turned to computational chemistry. The most stable isomer they found was a perfect hexagonal structure with one atom missing at its centre. A similar structure with two missing atoms was slightly less stable. They calculated the predicted photoelectron spectrum of the most stable cluster and compared it with the spectra that they had obtained in the experiment. They found generally good agreement, which suggests that the most stable isomer was the one they had in fact seen. Minor deviations between the theoretical and observed spectra could be explained by the presence of some two-hole isomer structures in the samples.
The isolated B36– nanoparticle is not perfectly flat. However, if the atoms at the corners were removed to create further holes along the joins, this molecule would then become a section of the α-sheet that was predicted in 2007. Wang and colleagues have dubbed this material borophene. Tantalizingly, the delocalized nature of boron–boron bonding suggests that it could be fully metallic, which might make it an even better electrical conductor than graphene. Wang emphasizes, however, that their computer models cannot predict whether or not it would have the distinctive property that gives graphene such extraordinary electron mobility: the transport of electrons as though they were massless particles. Nor can the researchers tell chemists how to produce the structure.
Cannot exist in nature
Not everyone agrees with the team’s conclusion regarding the α-sheet. The crystallographer Artem Oganov points out that calculations done by his group at Stonybrook University in the US and colleagues in China suggest that the α-sheet cannot exist in nature. In a upcoming paper in Physical Review Letters, the team uses a new, highly successful algorithm for structural prediction developed by Oganov’s group to show that the α-sheet structure is “so unstable it will spontaneously do something to increase its thickness by putting more atoms on top of itself”.
Among the more stable phases, the team calculated, would be a buckled bilayer structure called Pmmn-boron. Oganov and colleagues also calculate that electrons would travel in this material according to the Dirac equation, effectively making them massless particles. If confirmed, such a material would be the first planar “Dirac semimetal” not to have a graphene-like atomic structure, and the first in which the electronic conductivity within the plane was dependent on direction.
Oganov points out that his group’s own results do not directly contradict those of Wang’s team, as the latter claims only to have detected isolated B36– nanoparticles, not the complete α-sheet. “I have not looked at boron nanoparticles,” Oganov says, “but now I will, because I’m curious.”
Wang’s paper is published in Nature Communications. A preprint of Oganov’s paper is available on arXiv.