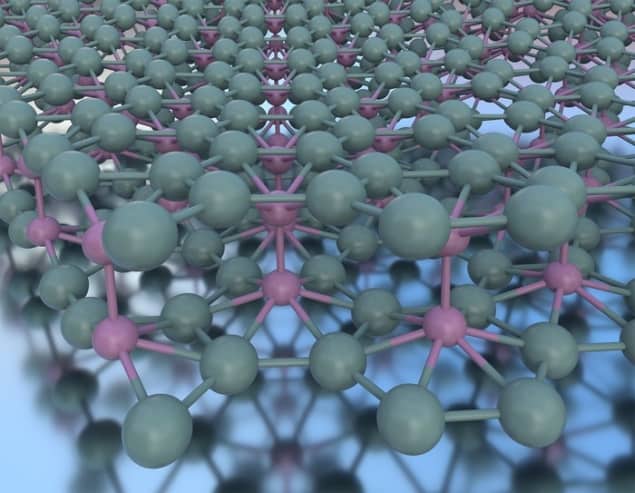
For the first time, a stable sheet of double-layered borophene has been created. The feat was achieved by a team co-led by Mark Hersam at Northwestern University and Boris Yakobson at Rice University, who created the material by accident. Their discovery could lead to the development of new technologies based on borophene, which is a 2D material like graphene.
Boron is one of the lightest and most chemically versatile elements on the periodic table. In 2015, Hersam colleagues discovered that the element can exist in flat, atomically-thin sheets dubbed borophene. Six years on and the material is now known to possess both electrical and mechanical properties that could rival those of carbon-based graphene.
However, unlike graphene, which can be easily made by peeling sheets from graphite, borophene sheets must be grown directly on a metal substrate – making the material much more difficult to fabricate. Indeed, researchers had been unable to prepare borophene in its double-layered form because the material would revert to its 3D bulk structure.
Flat terraces
In their study, Hersam, Yakobson and colleagues were investigating how the growth of single-layer borophene is affected by the properties of metal substrates. Initially, their experiment involved heating a silver sample to extremely high temperatures. This led to the formation of flat terraces on the surface, each several microns across and separated by single-atom steps.
As borophene grew on top of this substrate, a combination of microscopy techniques revealed that in some cases, a single borophene layer can spread beyond the boundaries of its terrace to cover the layer growing on an adjacent lower terrace. There, the material formed two bonded borophene layers: separated by roughly three atomic widths, which did not revert to the bulk structure.
Graphene-like boron is stabilized by hydrogen, paving the way for practical applications
While these sheets retained the highly desirable electrical and mechanical properties of single-layer borophene, the researchers propose that the space between the layers could be incredibly useful for energy or chemical storage. For example, the inclusion of a layer of lithium ions could enable the manufacture of highly advanced 2D batteries.
While the widely varied geometries of carbon nanotubes and multi-layered graphene have been widely explored so far, the team believes that borophene could display an even richer diversity of structures. In future experiments, they aim to explore these possibilities in more detail. Structures that they plan to explore include double layers with alternative atomic arrangements; and thicker borophene sheets, featuring three or more bonded layers. If materials with desirable properties are found, it could lead to new advances in fields as wide-ranging as electronics, sensing, solar energy, and quantum computing.
The research is described in Nature Materials.