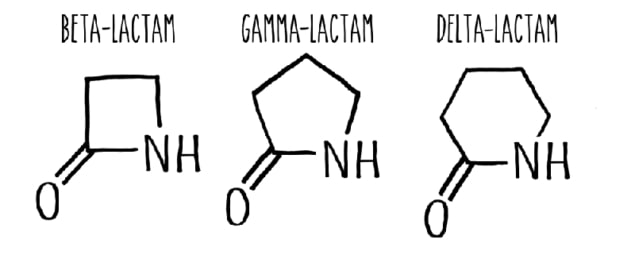
Enzymes can not only learn from synthetic chemistry but can also enable new chemistry that has never been realized before. So say researchers at the California Institute of Technology who have repurposed cytochrome P450 enzymes to produce β-lactams in a process called carbon-hydrogen (C-H) functionalization. The enzymes can also control which C-H bond in a substrate is functionalized, thus delivering other types of lactams, such as γ or δ-lactams. These molecules are important building blocks in organic synthesis and β-lactams are especially important in the pharmaceuticals industry – for example, to produce antibiotics such as penicillin.
C-H functionalization is one of the most sought-after strategies in synthetic chemistry. Since organic molecules often contain multiple similar C-H bonds, it is challenging to precisely control where along a molecule a reaction takes place. A promising approach is to make use of enzymes – the catalysts of the biological world – which are very site-selective for when it comes to biochemical transformations.
Cytochrome P450
Researchers led by Frances Arnold have been studying cytochrome P450, a class of heme-containing terminal oxidase enzymes that oxidize C-H bonds to C-O bonds. In particular, they have been focusing on a natural cytochrome P450 from Bacillus megaterium (P450BM3) and how to repurpose it to accommodate new-to-nature chemistry.
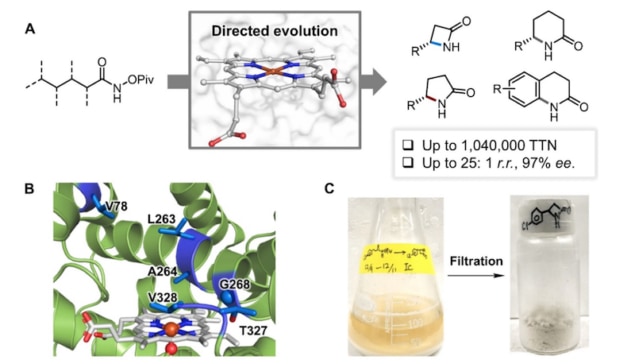
In their latest work, they employed a substrate containing a nitrene precursor inspired by a natural intermediate in the biosynthesis of benzastatins, and provided it to engineered versions of P450BM3. “Most of the enzymes we used didn’t do anything productive with the substrate, but one made a small amount of β-lactam product,” explains team member Inha Cho. “We thus decided to engineer this parent enzyme.”
The P450BM3 constructs lactams through intramolecular C-H amidation, she says. “The substrate is a chain-like molecule with C-H bonds and a terminal nitrene precursor. The engineered enzymes use the precursor to form an iron-bound nitrene intermediate and loop it to a specific C-H bond. It is like taking one end of a string and tying it in a knot in the middle of the string.”
Credit: Caltech
“In traditional synthetic chemistry, we have to tack extra reactive groups onto molecules that we want to turn into β -lactams”, explains team member Zhi-Jun Jia. Without these, the knots will end up tied in varying spots, resulting in a mixture of large and small loops. This is not ideal when trying to manufacture a homogenous batch of antibiotics. What is more, adding these extra reactive functional groups only complicates the synthesis by introducing additional steps.
Engineered enzymes target a specific C-H bond
The Caltech researchers engineered several enzymes from the same parent in a process called directed evolution, a technique that Arnold developed in the 1990s and for which she received the 2018 Nobel Prize in Chemistry. Here, the genetic code of a useful enzyme is transferred into bacteria like Escherichia coli and the bacteria then start producing this enzyme as they grow and divide.
They found that each of their engineered enzymes could target a specific C-H bond to produce lactams with different ring sizes. One of their nitrene substrates (an “acyl-protected” hydroxamate) has three sets of reactive C(sp3)-H bonds and the enzyme variants LSb, LSg and LSsp3 formed β-, γ- and δ-lactams respectively by selecting specific C-H sites on the substrate. The γ-lactam is made up of a loop of four carbon atoms and one nitrogen atom while the δ-lactam, a loop of five carbon atoms and one nitrogen.
The engineered enzymes are also very efficient, says Jia, with each enzyme molecule producing up to 1 million lactam products.

Frances H Arnold, George P Smith and Gregory P Winter win the Nobel Prize for Chemistry
“Our work shows that enzymes can innovate novel chemistry and help address important challenges in synthetic chemistry,” he tells Physics World. “We believe that they could realize many new-to-nature and even new-to-humankind reactions in the future. The technique we have developed could even help in the discovery and production of new antibiotics and pharmaceuticals, something that will be important especially as antibiotic resistance is becoming an increasingly important problem.”
The researchers, reporting their work in Science 10.1126/science.aaw9068, are now looking into industrial applications for their technology. “We will continue studying heme-containing enzymes, but are also trying to exploit non-heme ones to enrich our toolbox,” they say. “We hope that chemists will be able to use enzymes for synthesis in the future in the same way as they use small-molecule catalysts today.”