Elastic polymers can be stretched and released repeatedly without tearing and are widely employed in applications from disposable gloves to heart valves. Their main drawback is that they can generally be made either stiff or tough, but not both at the same time. A team at the Harvard John A Paulson School of Engineering and Applied Sciences (SEAS) has now developed an elastic polymer, or elastomer, that has both properties. The new material could find use in applications such as tissue regeneration, bioadhesives, bioprinting, wearable electronics and soft robots, and might also help reduce plastic pollution by increasing the durability of products made with elastomers.
Polymers are fabricated by linking together building blocks of monomers into chains. To make elastic polymers, these chains are crosslinked by covalent chemical bonds. These crosslinks connect the polymer chains so that when a force is applied to stretch the elastomer, the material recovers its shape when the force is removed. The more crosslinks, the shorter the polymer chain and the stiffer the material. This is because the energy stored in the materials decreases, making it brittle.
Entanglement bonds
Led by Zhigang Suo, the researchers made their new polymer simultaneously stiff and tough by using physical rather than chemical bonds. These types of bonds are known as entanglements, and have been known about for a long time, but until now they were only thought to affect polymer stiffness, not toughness.
In their work, Suo and colleagues fabricated a polymer in which all chains are long and cross-links are outnumbered by entanglements. The latter act as slip-links and stiffen the polymer. Unlike cross-links, though, they do not make the polymer brittle. This means that when an entangled polymer is stretched, the applied tension is transmitted along one chain and then to many other chains before a chain breaks.
“Crowded” monomers
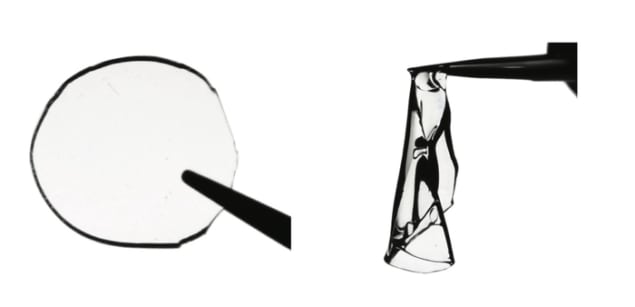
The researchers studied two polyacrylamide hydrogels – 3D polymer networks that can hold a large amount of water. They found that they could introduce many entanglements into the material using a precursor made of a concentrated monomer solution that contained 10 times less water than usual. The monomers are thus “crowded” into the solution, which forces them to become entwined like a tangled string of yarn, explain Junsoo Kim and Guogao Zhang, the study’s co-first authors. “Just like with knitted fabrics, the polymers maintain their connection with one another by being physically intertwined,” they say.

Polymer gels snap and jump on their own
By adding enough entanglement into the polymer chains, the team found that the hydrogels they studied could become tough without losing any of their stiffness. Indeed, with hundreds of these entanglements, only a few chemical crosslinks are required to keep the polymer stable and prevent the polymer chains from disentangling, they say. The hydrogels also have low friction and high wear resistance, which increase the durability and lifespan of the polymer. This last point is important since it could help decrease the consumption of plastics, Kim and Zhang suggest.
Looking forward, Suo and colleagues tell Physics World that they are now busy developing highly entangled hydrogels using polymers suitable for biomedical applications.
The new study is detailed in Science.