Physicists have made a "complete" measurement of the break-up of a molecule for the first time. Reinhard Dörner of the University of Frankfurt and co-workers in Germany, the US, Australia and Spain recorded the two electrons and two nuclei that were released when a single photon split a molecule of deuterium into its basic components (T Weber et al. 2004 Nature 431 437). The technique could help shed new light on the fundamental properties of molecules.
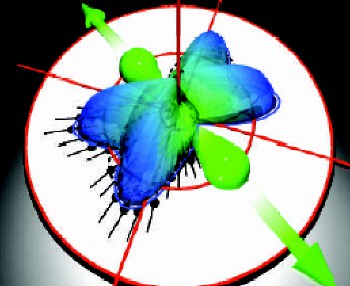
In the experiments a single photon has enough energy (75.5 electron volts) to knock both electrons out of a deuterium molecule, and the two nuclei then fly apart because they are both positively charged. By measuring the momenta of all four particles it is possible to learn more about what was happening inside the molecule.
Dörner and co-workers started by ionising a jet of deuterium molecules with polarised photons from the Advanced Light Source at the Lawrence Berkeley National Laboratory in the US. They used deuterium instead of ordinary hydrogen because it is heavier and therefore provides a higher target density for the photon beam. (A deuterium nucleus contains a proton and a neutron whereas a hydrogen nucleus contains just a proton.)
Next, they used electric and magnetic fields to accelerate the electrons created in the ionisation process in one direction, and the nuclei in another. The particles then left the region containing the electric field and drifted onto “micro-channel plate” detectors. For each particle, Dörner and co-workers were able to measure how long it took to reach the detector and the position of impact on the plate. This allowed them to calculate the initial momentum of all four particles and build up a 3D image of the photo-fragmentation “explosion” (see figure).
The results show that the behaviour of the electrons is strongly influenced by the separation of the nuclei at the instant the photon is absorbed. The experiment could lead to a better understanding of many physical and chemical processes through improved knowledge of the quantum dynamics of many-particle systems.



