The “wonder material” graphene burst onto the scene five years ago — and the sheet of carbon just one atom thick continues to wow physicists with its growing list of remarkable properties. Now, a team including the UK-based research group that discovered graphene has created a new material called graphane by adding hydrogen atoms to their original discovery.
As well as being an insulator that could prove useful for creating graphene-based electronic devices, graphane might also find use as a hydrogen-storage medium that could help hydrogen-powered vehicles travel further before refuelling.
Despite being extremely thin, graphene has great physical strength as well as being an excellent conductor of both heat and electricity. What’s more, it is a semiconductor with electronic properties that can be adjusted by simply applying a voltage across a region of a graphene sheet — rather than by introducing chemical impurities as in silicon. As a result, some researchers believe that it could be used to create transistors that are smaller and faster than silicon-based devices.
Further on a tank of graphane?
Thanks to its low mass and large surface area, graphane has also been touted as an an ideal material for storing hydrogen fuel on vehicles. Finding an economical way of storing enough hydrogen for a reasonably long journey is a major challenge because liquefying the gas (as is done with propane)is prohibitively expensive in terms of both money and energy.
However, making graphane had proven to be difficult. The problem is that the hydrogen molecules must first be broken into atoms and this process usually requires high temperatures that could alter or damage the crystallographic structure of the graphene.
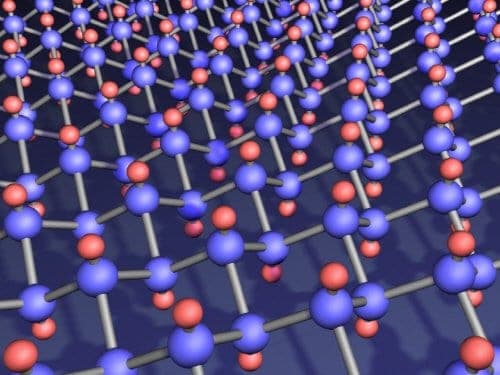
Now, a team led by Andre Geim and Kostya Novoselov at the University of Manchester has worked out a way to make graphane by passing hydrogen gas through an electrical discharge. This creates hydrogen atoms, which then drift towards a sample of graphene and bond with its carbon atoms.
The team studied both the electrical and structural properties of graphane and concluded that each carbon atom is bonded with one hydrogen atom. It appears that alternating carbon atoms in the normally-flat sheet are pulled up and down — creating a thicker structure that is reminiscent of how carbon is arranged in a diamond crystal. And, like diamond, the team found that graphane is an insulator — a property that could be very useful for creating carbon-based electronic devices.
Fine tuning graphene’s properties
“This is the first step in being able to fine tune the electronic properties of graphene by attaching various species to its scaffolding,” said Novoselov. “It is a new look at graphene, if you like, with far reaching and promising consequences.”
The next step would be to learn how to control the electronic properties by adding other chemicals, with perhaps different arrangements of these species on graphene’s surface, according to Novoselov.
“The modern semiconductor industry makes use of the whole periodic table, from insulators to semiconductors to metals. But what if a single material could be modified so that it covers the entire spectrum needed for electronic applications?” added Geim. “Imagine a graphene wafer with all interconnects made from highly conductive, pristine graphene whereas other parts are modified chemically to become semiconductors — and work as transistors — or become insulators.”
And that’s not all: the team also showed that reaction is reversible because graphane can be converted back into graphene by heating it up so that the hydrogen is removed. This property, plus the high hydrogen density and low mass of graphane, could make it a candidate for hydrogen storage.