Myelin is a protective layer that forms around nerves to insulate them and speed transmission of electrical impulses. Demyelination, loss of this insulating layer, contributes to many neurological diseases, including multiple sclerosis, Alzheimer’s disease, stroke and dementia. An effective technique to detect this potentially reversible condition could improve diagnoses of brain diseases and enable monitoring of possible treatments. Currently, however, no imaging tests can accurately identify demyelination.
To address this shortfall, researchers from the Gordon Center for Medical Imaging at Massachusetts General Hospital and Harvard Medical School are investigating the use of a novel PET radiotracer – 18F-3-fluoro-4-aminopyridine (18F-3F4AP) – to image demyelinated lesions in the brain. They have now tested the tracer in humans for the first time, reporting their findings in the European Journal of Nuclear Medicine and Molecular Imaging.
“Having an imaging tool that it is specific to demyelination can help to better understand the contribution of demyelination to different diseases and better monitor a disease or the response to therapy – for example, a remyelinating therapy,” says first author Pedro Brugarolas in a press statement.
18F-3F4AP is a radiofluorinated version of the multiple sclerosis drug 4-aminopyridine. The tracer, which enters the brain via passive diffusion, binds to demyelinated axons in a similar manner to the drug itself. Previous studies demonstrated that PET with 18F-3F4AP can detect lesions in a rat model of demyelination, and that the tracer has suitable properties for imaging the brains of rhesus macaques, prompting the team to investigate its use in humans.
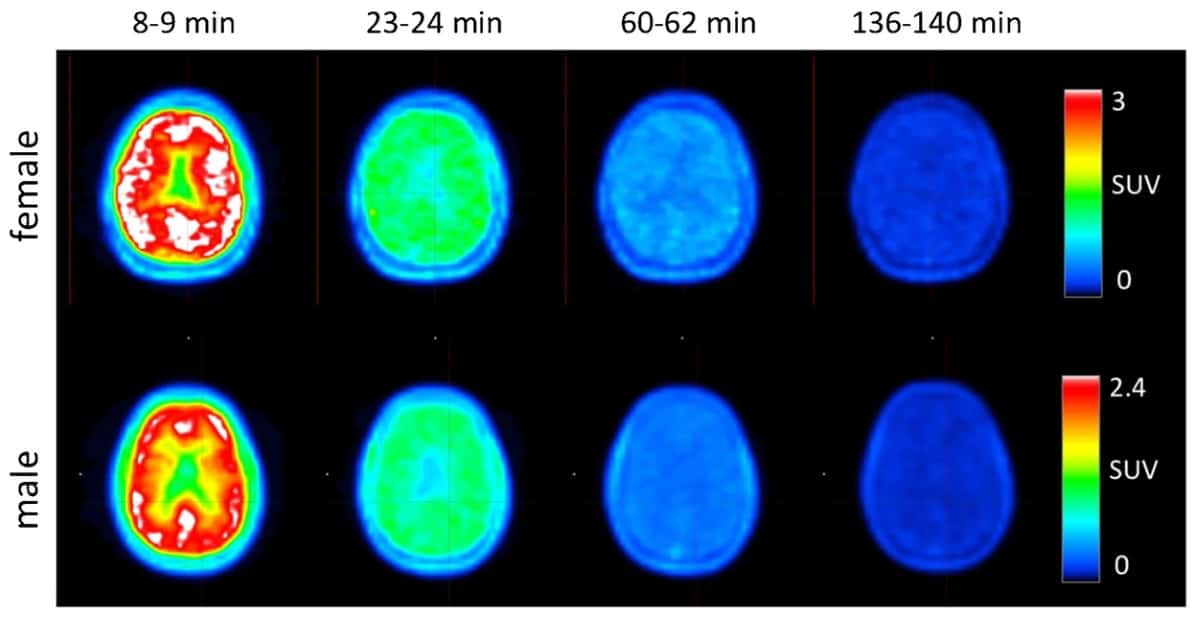
Brugarolas and colleagues performed PET scans on four healthy volunteers after administering 368±17.9 MBq of 18F-3F4AP. Following a low-dose CT scan, they started PET immediately upon tracer injection, recording a series of images in seven scanner bed positions to cover the entire body. To capture the tracer kinetics and maximize image quality, the initial scan time per position was 1 min, increasing to 2, 4 and 8 min per position. The entire PET acquisition took 4 h.
The resulting PET images and time-activity curves (TACs) revealed that the tracer distributed rapidly throughout the entire body, including the brain, and quickly cleared via renal excretion. At 8–14 min post-injection, maximum activity was seen in the liver, kidneys, urinary bladder, spleen, stomach and brain. At 22–28 min, the highest activity was in the kidneys, biliary duct and urinary bladder. After 60 min, most of the activity had cleared from the organs and accumulated in the urinary bladder.
The team also used the integrated TACs to perform dosimetry. The average effective dose was 12.2 ± 2.2 µSv/MBq for the four participants, with no differences seen between male and female volunteers. The researchers note that this effective dose is significantly lower than that estimated from non-human primate studies (21.6 ± 0.6 µSv/MBq), likely due to the faster clearance seen in humans than in rhesus macaques. This dose was also lower than for other PET tracers, such as 18F-FDG.
Importantly, the tracer and imaging procedure were well tolerated by all participants, with no adverse events occurring during the scan. There were no significant differences in volunteers’ vital signs (temperature, blood pressure and oxygen saturation) before and after the scan, and no significant changes in blood metabolite and electrocardiogram results obtained within 30 days before and after the scan.

PET tracer measures demyelination in mice
The researchers conclude that 18F-3F4AP readily enters the brain and is safe in for use in humans, with an acceptable level of radiation dose. They suggest that their findings open the door for further studies investigating the tracer’s ability to detect demyelinated lesions in different patient populations.
Brugarolas tells Physics World that the team is currently pursuing two small clinical studies using the new tracer: to investigate its value for imaging multiple sclerosis; and to assess its use in patients with traumatic brain injury, mild cognitive impairment and Alzheimer’s disease.