
Researchers from National Tsing Hua University in Taiwan have used an injectable self-assembling hydrogel to treat brain injury. The nanopeptide hydrogel (RADA16) promoted neurovascular growth in the lab, as well as regenerating damaged neurovascular tissue in a zebrafish model of brain injury (Nanoscale 9 16281).
Hydrogels are biomaterials that are highly saturated with water but have the physical properties of a solid. The biocompatibility and physical properties of hydrogels enable encapsulation and growth of cells in the lab, or in some cases, injection directly into the brain to aid recovery and regeneration.
RADA-peptide hydrogel
The RADA16 (peptide sequence RADA, with 16 repeats) hydrogel used in this study can be tuned physically for the intended application. To enable cells to attach to RADA16, an additional peptide sequence (SVVYGLR) is added to the hydrogel. Cellular attachment is important for cell proliferation and to translate external physical stimuli into alterations of gene and protein levels within the cell.
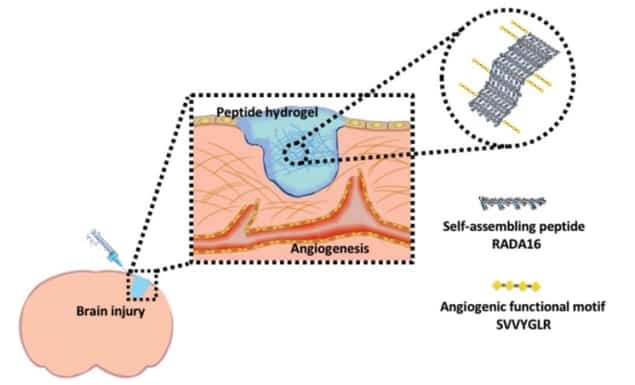
The group showed that the RADA16-SVVYGLR hydrogel enabled angiogenesis (new blood vessel growth) and neurogenesis (increased neural growth) both in vitro and in vivo. These findings validate this biomaterial as a promising option for tissue engineering and disease modelling in the lab, and for treatment following brain injury.
Zebrafish model
For the in vivo study, the researchers created a wound in a specific region of the zebrafish brain, and subsequently injected RADA16-SVVYGLR into the wound site. Not only did this promote neurovascular regeneration, it also proved beneficial to the functional recovery of the zebrafish (as demonstrated in a series of cognitive behavioural tests).
The success of this hydrogel in the zebrafish model is encouraging, as a lot of naturally produced hydrogels (for example collagen) have been shown to cause adverse tissue–tissue adhesions when injected, damaging the surrounding tissue.
Physical properties and tuning
The authors note that the physicochemical properties of the hydrogel were easily tuned, with both pH and the concentration of nanopeptide altering the micro-environmental architecture surrounding the cells. This is important as different tissues in the body require different physical properties, such as mechanical stiffness, for example. Importantly for neurovascular tissue, the hydrogel could be tuned to lie within a suitable stiffness range for neurovascular development.
An additional advantage of the RADA16-SVVYLGR peptide sequence is that its physical charge allows the hydrogel to assemble into ordered β-sheets without any modifications; unlike many other hydrogel formulations, which require potentially damaging chemical processes to form solid structures.
This feature – along with the tuneable properties – enables potential optimization for 3D bioprinting, which would allow the development of layered tissue models or bio-fabricated tissues and organs for transplants.
Bench to bedside translation
The favourable results produced in vivo for neurovascular regeneration imply that this system could be utilized in clinics for treatment of traumatic brain injury, stroke or neurodegenerative diseases. Additionally, the in vitro results could lead to the use of this nanopeptide for neural tissue engineering and tissue modelling applications.



