A new type of stent with “pop-up/fold-down” needles that deliver drugs to tubular organs such as the gastrointestinal tract, vasculature and airway has been designed by a team of US-based engineers and physicians. The stent’s flexible design was inspired by kirigami, the Japanese art of folding and cutting paper to create three dimensional structures, and by the scaly skin of snakes and sharks.
The kirigami-based injectable stents, described in Nature Materials, could serve as a new class of implantable drug-releasing systems, capable of deploying deposits of drugs in multiple locations. In particular, the stents are designed to improve localized drug delivery for diseases that affect tubular organs such as the oesophagus and bowel.
Tubular structures in the body, particularly vertically oriented or winding organs, are difficult to coat with therapeutics. Diseases in these areas are currently treated with a broad range of approaches including topical drugs and/or peripheral injections, although the drug may spread to other parts of the body causing side effects.
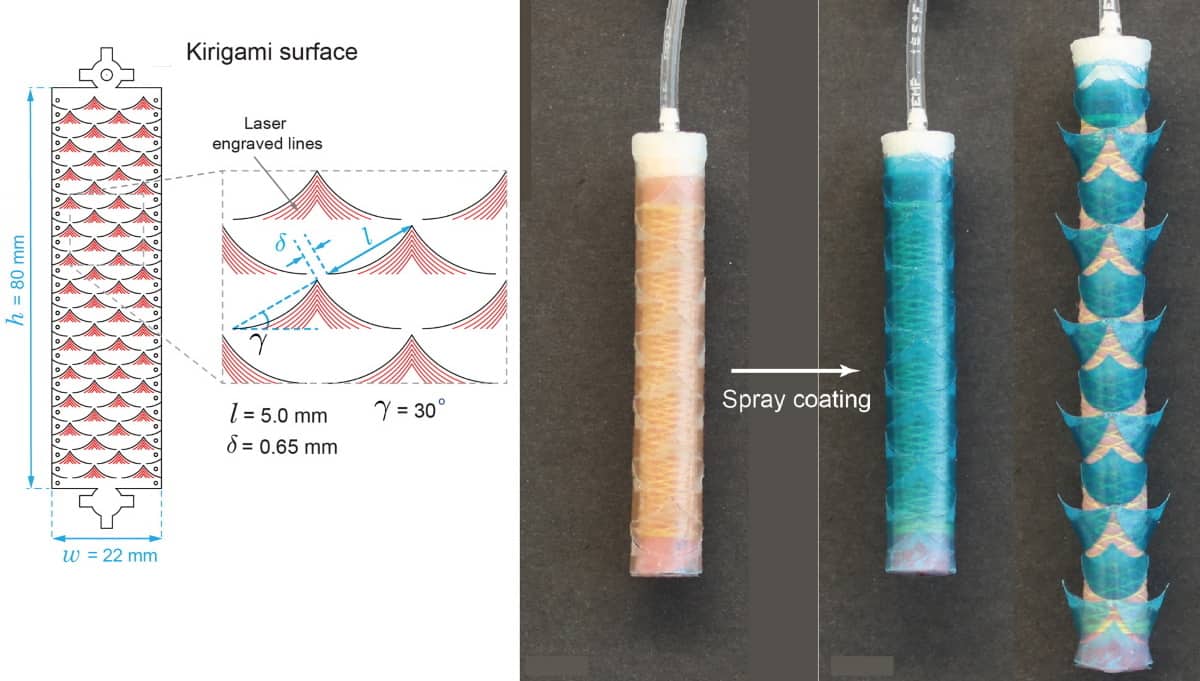
To address this shortfall, the multidisciplinary team – from Brigham and Women’s Hospital, Massachusetts General Hospital and Massachusetts Institute of Technology (MIT) – created a flexible, cylindrical stent comprising a stretchable kirigami shell integrated with a fluidically driven linear soft actuator.
The cylindrical skin consists of a periodic array of snake-denticle-shaped cuts embedded in thin plastic sheets. The cuts form barb-shaped “needles” that pop up when the tube is pressurized and expands. These needles penetrate tissue, injecting drug-containing degradable microparticles that release the drugs over a period of time.
The actuator, made of 1.5-mm-thick silicone-based rubber, delivers controlled air pressure to expand the stent to up to 60% of its original diameter and lodge it firmly in the organ structure. The air pressure forces the kirigami needles to buckle outward, changing their orientation from planar to perpendicular to the stent body surface. After the microparticles are injected, the air pressure is released and the needles return to their original flat configuration for easy and safe stent removal.
Cut and fold
Kirigami-inspired design is not new to medicine. It has been used to create bandages that stick more securely to joints and to create lightweight sensors that monitor joint motion. Senior author Giovanni Traverso, who is both a gastroenterologist and a biomedical engineer, says that his lab at MIT has previously designed and tested buckling-induced kirigami surfaces for the soles of shoes to increase friction forces and reduce slipping on slick surfaces.
“This technology of our stent could be applied in essentially any tubular organ,” Traverso explains. “Having the ability to deliver drugs locally, on an infrequent basis, really maximizes the likelihood of helping to resolve patients’ conditions and could be transformative in how we think about patient care by enabling local, prolonged drug delivery following a single treatment.”
The researchers created kirigami needles of several different sizes and shapes for their study. By varying those features, as well as the thickness of the outer plastic sheet, the researchers could control the depth to which the needles penetrate into tissue. They tested the stents by endoscopically inserting them into the oesophagus of three Yorkshire pigs weighing between 50 and 80 kg. Pigs have similar anatomical dimensions to adult humans and have been widely used to evaluate biomedical gastrointestinal devices.
Once in place, the researchers inflated the balloon inside the 8 cm long and 12.5 mm diameter stent, allowing the needles to pop up and penetrate about half a millimetre into the tissue. They deflated the balloon two minutes later, after the microparticles were deposited, flattening the needles to enable the stent to be endoscopically removed.
Low-cost kirigami sensor tracks shoulder movement
The microparticles contained budesonide, a steroid used to treat inflammatory bowel disease and eosinophilic esophagitis. The researchers measured its release at one, three and seven days following injection. They were able to detect the drug even seven days after delivery. “This indicates rapid absorption of the drug in the tissue, enabling sustained local delivery of budesonide and supporting the potential for this controlled drug-releasing system to deliver drug agents to tubular segments of the GI tract,” they write.
“As part of our ongoing work, we are aiming to develop systems that can deliver drugs for multiple months for a range of conditions, with a goal of providing the needed treatment after a single administration event,” Traverso tells Physics World. “We view these approaches as having the capacity to transform the patient experience by reducing the need to take medications and thereby significantly improving drug adherence.”