
Although nearly all ice found on Earth has a hexagonal structure, at least 17 types of ice are known to exist, each with a different molecular arrangement. Most of these novel variants, however, require high pressures and controlled temperature environments to form, making them difficult to study directly. A team of researchers in China has now used a first-principles neural network potential technique to discriminate between several high-pressure water phases. Their findings add to our understanding of the proton transfer mechanisms involved when these phases melt and could prove important for planetary science as well as fundamental physics and chemistry.
Water is unique in forming a wide variety of different crystalline and amorphous ice structures. Its unusual behaviour in the frozen state stems in part from the weak intermolecular bonds between its two hydrogen atoms, which are separated by a single atom of oxygen.
One of the most-studied alternative forms of ice is known as ice VII. This exotic body-centred cubic (bcc) crystal phase is also known as “hot ice”, and it can form at ambient temperatures under pressures above 3 GPa (about 30 000 times atmospheric pressure at sea level). Ice in this form has been theorized to exist in cold subduction zones within the Earth’s crust, and on Saturn’s icy moon Titan.
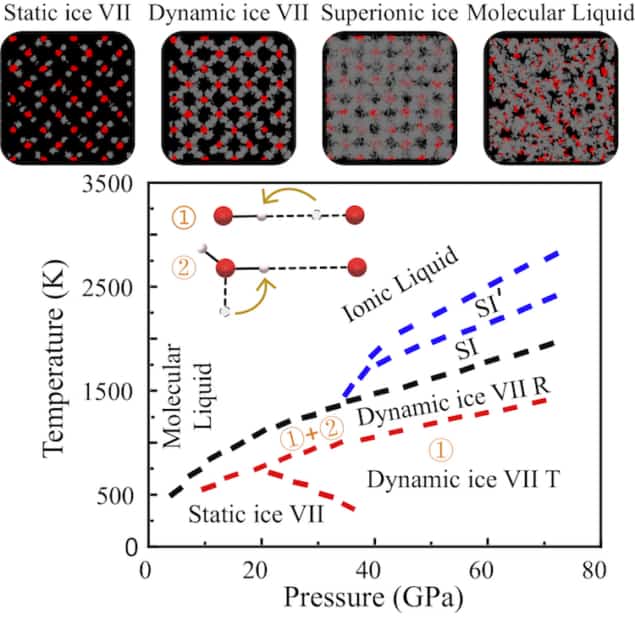
Another novel ice phase, known as superionic ice or ice XVIII, exists at even higher temperatures and pressures of 1000 kelvin and 40 GPa. This form of frozen water contains liquid-like hydrogen ions – that is, protons – that quickly diffuse through a solid lattice of oxygen atoms. Superionic ice could make up a large fraction of the interiors of the planets Uranus and Neptune, with the fast-diffusing protons helping to generate the strong and complex magnetic fields characteristic of these “ice giant” planets.
Ice VII to superionic ice
In 2005, researchers confirmed experimentally that ice VII can transform into superionic ice at around 47 GPa and 1000 K. They also suggested that the transformation takes place via a “dynamic” form of ice VII in which the protons are more disordered than in conventional ice VII, while still being more localized than they are in superionic ice. This transition, however, proved difficult to simulate using traditional ab initio calculations and force field molecular modelling methods. This is because ab initio methods are normally restricted to short time periods and relatively small supercell simulations, whilst force field-based techniques cannot address the chemical bond breaking associated with proton diffusion in dynamic ice VII.
Xin-Zheng Li of Peking University in Beijing and colleagues believe they have now overcome this problem by applying an alternative modelling technique based on neural network potentials. Their model used a popular deep-learning package (the DeePMD-kit) to create large-scale simulations that are as accurate as density-functional theory (DFT) calculations and require about the same amount of computing time and power.
The results of these simulations enabled the researchers to explore the nature of high-pressure water phases at an atomic level. They found that dynamic features, such as the diffusive motion of hydrogen and oxygen atoms, are indispensable for distinguishing between several subtle and “non-trivial” ice phases.
Detailed phase diagram
In dynamic ice VII, for example, Li’s team identified two subtle phases, which they dubbed dynamic ice VII T and dynamic ice VII R. The former incorporates local motion and transversal transfer of protons while the latter also accounts for their rotational transfer. They also interpreted the superionic phase as involving the non-trivial melting of ice VII at pressures above 40 GPa.

New superionic ice phase could shed more light on icy giant cores
According to Li, the transition from ice VII to superionic ice can be understood as occurring when oxygen and hydrogen atoms simultaneously escape from the crystal sites in ice VII at moderate pressures just as the solid structure suddenly breaks down. At higher pressures (above 40 GPa), the hydrogen atoms melt first, followed by the oxygen atoms.
Based on these results, the researchers have drawn up a detailed phase diagram (see image above), which they say will be useful for understanding how water behaves under high pressures. “The method to detail this diagram could be extended to other materials – even those that are thought to be relatively well understood,” Li tells Physics World.
The research is detailed in Chinese Physics Letters, which is published by IOPP.



