This year has been a year like no other. In 2020, many physicists turned their research efforts towards tackling the pandemic. Within medical physics, researchers worked to develop improved diagnostics and potential treatments for COVID-19, as well as coming up with innovative technologies and devices to help healthcare workers.
Alongside, the medical physics community continued to innovate in the more traditional areas of research – from new approaches to delivering radiotherapy to novel diagnostic imaging devices and techniques. Here are a few of the highlights that caught my eye.
Tumour hypoxia tracked in real-time
Hypoxia, or lack of oxygen, in a tumour can make it resistant to radiotherapy. But currently, there’s no method to monitor tumour oxygenation noninvasively or without averaging across the whole lesion. In January, a team headed up at Dartmouth-Hitchcock’s Norris Cotton Cancer Center demonstrated that Cherenkov excited luminescence imaging (CELI) could be used to non-invasively image oxygen distribution in tumours during radiation delivery.

Brian Pogue and his team irradiated tumours in mice with X-rays, generating Cherenkov light that served as an internal source to excite a phosphorescent probe. They examined two tumour lines, one that’s responsive to radiation and one that’s radioresistant, and saw differences in tumour oxygenation that reflected their differences in response.
The researchers concluded that time-resolved CELI offers high spatial resolution and could be easily added to clinical protocols to evaluate tumour oxygenation at the time of radiation delivery – a long-sought goal in cancer therapy.
Real-time dosimetry for FLASH radiotherapy
Ultrahigh dose rate radiation therapy (FLASH) is a hot topics in radiotherapy research. Animal studies have shown that FLASH can destroy tumours while vastly reducing damage to normal tissue, and clinical trials are just beginning. But with such high dose rates, it is vital to continuously monitor dose deposition in the patient. Researchers at the University of Michigan have proposed a new technique, ionizing radiation acoustic imaging, that can measure dose while simultaneously obtaining images of the radiation target and surrounding tissue.
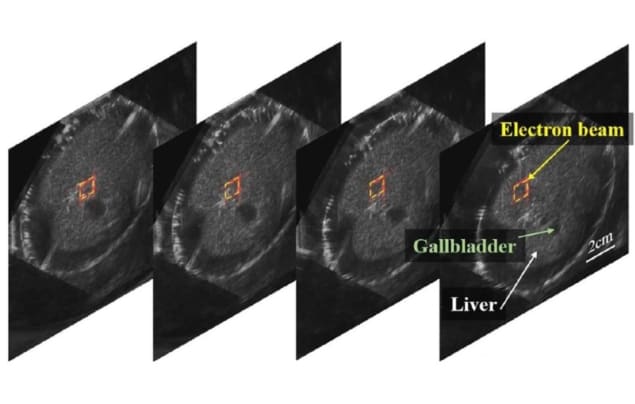
The method is based on a thermoacoustic effect: as ionizing radiation deposits energy in the patient, the tissue temperature increases, causing expansion and a propagating pressure wave. In conventional radiotherapy, these waves are extremely weak. But with FLASH dose rates (40 Gy/s or more), the signals can be detected by ultrasound probes on the patient’s skin.
First author Ibrahim Oraiqat and colleagues showed that this acoustic imaging signal increased linearly with the dose-per-pulse and that measurements at different depths agreed with those from commercial film dosimeters. They also demonstrated simultaneous acoustic dosimetry and ultrasound imaging in a moving rabbit liver phantom.
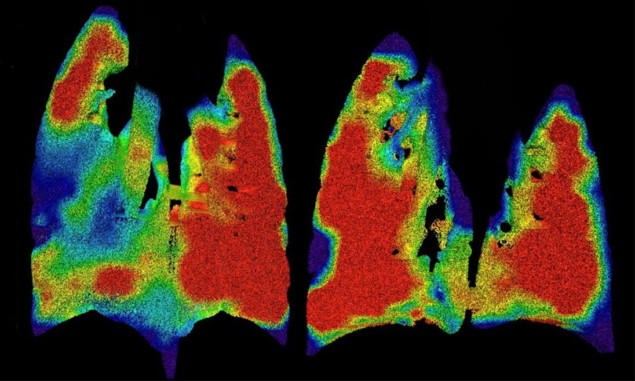
Imaging airflow through the lungs
Cystic fibrosis (CF) is a hereditary disease that causes sticky mucus to build up in the lungs, hindering breathing and leading to lung infections. The lung damage caused by CF is often non-uniform, and treating the patches of damaged tissue can slow disease progression. To quantify and localize such damaged regions, researchers in Australia have developed a novel tool to measure regional lung function.
The team, led by Freda Werdiger from Monash University, used X-ray velocimetry (XV) to non-invasively generate high-definition images of real-time airflow through the lungs of mice. XV combines high-speed imaging – in this case, propagation-based phase-contrast X-ray imaging – and post-processing analysis to produce a detailed ventilation map of the lungs. The researchers tested the technique in mice with CF-like lung disease and their healthy littermates. Maps of regional lung expansion clearly showed the presence and locations of areas of airflow deficits in the diseased animals. Following its recent commercialization, this technology could help improve the length and quality-of-lives of people with CF and other respiratory diseases.
Non-invasive skin cancer detection
Suspicious skin lesions are usually identified by dermatologists using a handheld optical magnifier, and subsequently diagnosed via pathological analysis of a tissue biopsy. This process, however, is invasive, costly, time-consuming and dependent upon the skill of the physician. To address these shortfalls, Abraham Katzir of Tel Aviv University and co-researchers are creating an accurate, affordable clinical system that can identify skin cancers in near-real-time.
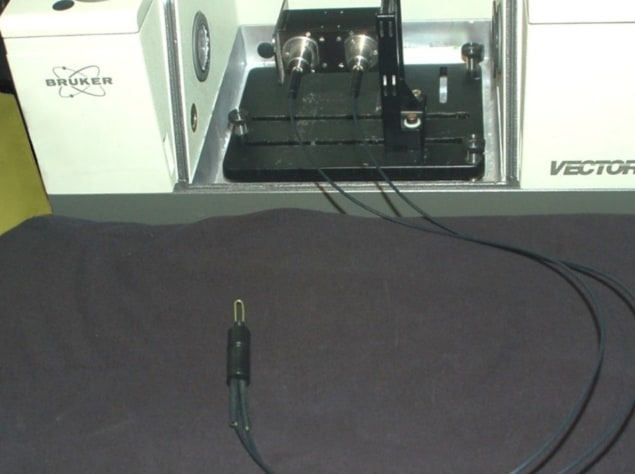
The team has developed a fibre-optic evanescent wave spectroscopy system based on a long, U-shaped, mid-IR transmitting fibre connected to a mid-IR spectrometer. By simply touching suspicious regions for 30 s with centre of the fibre, the system could identify cancerous lesions – including melanoma, basal cell carcinoma and squamous cell carcinoma – on patients’ skin.
Katzir suggests that in the future, this non-invasive “spectroscopic pathology” could replace standard invasive biopsies.
A portable brain MRI scanner
As 2020 drew to a close, we reported on a low-cost, portable brain MRI scanner being developed by researchers at Massachusetts General Hospital/Harvard Medical School. MRI is the standard modality for assessing neurological disorders, but conventional high-field scanners are expensive, immobile and require dedicated power and cooling infrastructure. As such, MRI is unavailable to critically ill patients who cannot be safely transported to the scanner or patients in low-resource settings.
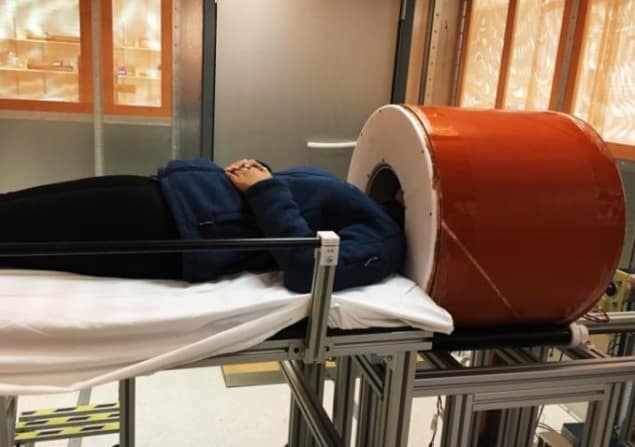
To address this, Clarissa Cooley and colleagues used an array of neodymium rare-earth magnets to generate an 80 mT static field. This permanent magnet does not require external power or cryogenic cooling, allowing the researchers to build a truly portable MRI brain scanner that can be wheeled around on a cart and operated from a standard power outlet. They demonstrated that the system could perform standard brain scans employed for diagnosing and monitoring clinically important brain pathology. Such a portable scanner could be used in many locations, such as a patient’s bedside, at the scene of an accident or in a rural clinic – expanding access to MR neuroimaging.



