Physicists in the US have made the surprising discovery that two nuclear isotopes with exactly mirrored numbers of protons and neutrons have different ground states. The researchers, led by Daniel Hoff and Andrew Rogers at the University of Massachusetts Lowell, uncovered this mirror symmetry violation by accident, while investigating the nuclear physics of astronomical X-ray bursts. Their discovery could have profound implications for our knowledge of the physics that govern atomic nuclei.
Many processes in particle physics have properties rooted in conservation laws, which describe how certain quantities must remain the same throughout nuclear reactions and decays. One effect that emerges from these laws is called “mirror symmetry” and relates to isotope “partners” whose numbers of protons and neutrons are exactly switched with each other. Such partners are known to have identical ground states in terms of angular momentum and parity. This reflects the similarity of the behaviors of protons and neutrons in the nucleus – something that underlies theories of nuclear physics.
In their study, Hoff and colleagues looked at strontium-73 (38 protons and 35 neutrons) and bromine-73 (35 protons and 38 neutrons). However, their original plan was to study the rapid proton capture exhibited by another isotope, rubidium-73. This process is thought to be involved in the production of some astrophysical X-ray bursts. Rubidium-73 is very short lived and the team were producing it via the beta-decay of strontium-73.
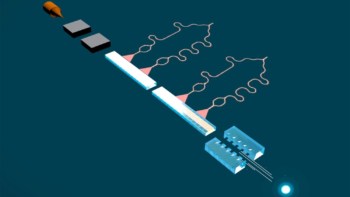
Stack of silicon detectors
Using the National Superconducting Cyclotron Laboratory facility at Michigan State University, the researchers first fired a beam of nuclei containing strontium-73 into a stack of silicon detectors. After being slowed to a halt, the nuclei decayed into rubidium-73 by emitting a single positron, followed by a proton, which are detected.
Looking at how the two particles are emitted from the isotope provided information about how rubidium-73 captures protons. However, their analysis led them to a wholly unexpected discovery: that the ground state of strontium-73 must be identical to that of rubidium-73. The result came as a surprise because strontium-73 is a mirror partner of another isotope, bromine-73, which is known to have a different ground state from the strontium-73 ground state determined by Hoff and colleagues. This means that strontium-73 and bromine-73 appear to violate mirror symmetry.
The researchers suggest that the violation could be the result of several factors including differences in shape between the two partners, but more experiments will be required to confirm their influences. These include direct measurements of strontium-73’s mass and also use beta nuclear magnetic resonance (β-NMR) to determine the ground-state spin of the isotope.
The team already has further experiments planned. The research is described in Nature.