The goal of mechanobiology is to develop a mechanical description of cells and their parts. Platelet clotting, for example, is an important biological process that can only be fully understood if a mechanical account of platelet dynamics is given. Typically, the first task when setting up a mechanics problem is to create a force diagram – a diagram of the magnitude and direction of all forces involved in a system. One challenge in mechanobiology, however, is that no current technique can construct a force diagram for molecular forces in cells.
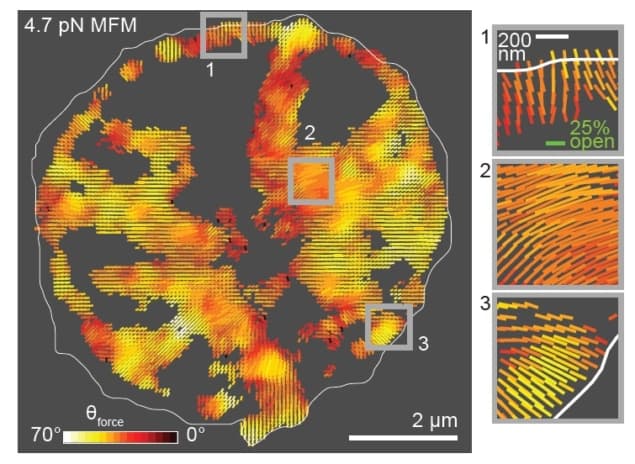
To address this limitation, a team of researchers at Emory University has introduced a technique called molecular force microscopy (MFM). MFM combines two techniques – molecular tension probes and fluorescence polarization microscopy – to measure the magnitude and 3D orientation of cellular forces. In their latest work, the authors discuss the details of MFM and demonstrate the technique’s usefulness by mapping forces in fibroblasts and human platelets (Nature Methods doi: 10.1038/nmeth.4536).
In a previous study (Nature Communications 5 5167), the team developed a molecular tension probe with piconewton resolution. These probes have three components: a fluorophore, a quencher, and an extendible, spring-like domain composed of a DNA hairpin.
The fluorophore and quencher sit at either end of the DNA hairpin. When in close contact, the quencher stops the fluorophore from fluorescing, but when the DNA is extended, the two separates and a strong fluorescence signal is produced. The intensity of this signal can be used to measure the magnitude of the force pulling on the probe.
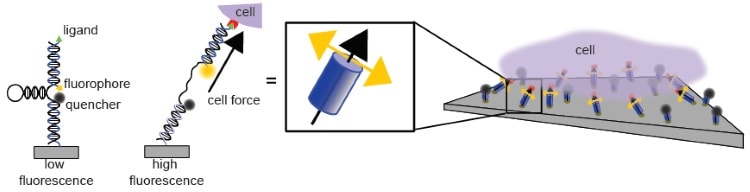
In their current work, Joshua Brockman and colleagues anchored a grid of these probes between an immobile surface and a network of receptors on the surface of a cell. When the receptors change configuration, they exert a tension force on the probe and extend the hairpin. Thus, with this network of probes, the researchers were able to create a map of the magnitude of piconewton tension forces exerted by cellular receptors.
To measure force orientation, the researchers used a technique called fluorescence polarization microscopy, which measures the orientation of a fluorophore by exciting it with light polarized at a series of distinct angles. Because each DNA-based tension probe contained a fluorophore, the team was able to estimate the orientation of the force by measuring the orientation of the fluorophore.
The researchers applied MFM to two systems: fibroblasts and human platelets. In both cases, the technique revealed an alignment between the organization of force-bearing structures and their force vectors that had not been seen before.
“Next, we plan to use this technique to understand how different biomolecules contribute to transfer mechanical signals into cells. We’re removing different proteins from cells and observing the effects those removals have on force orientation”, says co-author Aaron Blanchard. The team hopes that this technique will help the research field develop a broader understanding of the role of mechanical forces in biochemical networks of cellular signalling.



