
Identifying anatomic changes shown on MRI scans during a course of pencil-beam proton therapy, and adapting treatment plans accordingly, could improve the treatment quality, effectiveness and safety. In a retrospective study, researchers at St. Jude Children’s Research Hospital determined that 27% of paediatric patients, the majority with brain tumours, would have benefited from mid-treatment plan changes.
Pencil-beam proton therapy delivers radiation doses to a target with high accuracy, but if the target shifts, the dosimetric advantage afforded by the Bragg peak may be compromised and the risk of toxicities to adjacent tissue increased. Prior clinical trials comparing outcomes of paediatric patients treated with either proton or photon therapy have produced mixed results with regard to toxicity.
The researchers hypothesized that adherence to the treatment plan without adjusting for anatomic changes may have compromised the ability of proton therapy to maximally spare normal tissue while adequately covering the target volume. As such, they investigated whether altering treatment plans to compensate for changes in tumours or adjacent organs-at-risk (OARs) could improve target dose delivery and reduce normal tissue dose. They published their findings in the International Journal of Radiation Oncology, Biology, Physics.
The study included 73 children with a variety of cancers who received proton therapy at St. Jude during a 30-month period starting January 2017. The patients had between one and seven MRI exams, most of which were performed between weeks two and four of their treatments. Children with low-grade gliomas or rhabdomyosarcomas underwent weekly MRI exams.
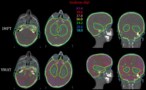
The researchers evaluated 230 MRI scans acquired in the treatment position during the patients’ proton therapy courses, recording patterns of temporal and spatial anatomic changes. To identify scenarios that would benefit significantly from re-optimization, they compared the calculated radiation dose for the anatomy-of-the-day without treatment adaptation with the dose that would have been delivered with an adapted plan.
The majority of patients had brain tumours (79%) or tumours located in the head-and-neck (12%). They were prescribed a median dose of 54 Gy(RBE) in 1.8 Gy(RBE) per fraction. MRI analysis revealed that 20 patients had anatomic changes in their gross tumour volume (GTV) and/or changes in the tissue density within the beam path. This included all eight patients with rhabdomyosarcomas and 24% of patients with low-grade gliomas.
Led by radiation oncologist Sahaja Acharya, the researchers determined that 11 of the 20 re-optimized treatment plans had significantly changed plan quality, defined as a 5% or greater decrease in CTV V95 (clinical target volume receiving at least 95% of the prescription dose) or a 5% or greater increase in the dose–volume parameter used as an OAR constraint.
Because of anatomic changes, seven patients experienced a significant reduction in CTV coverage with the delivered plan, ranging from 5–16% decrease in CTV V95. Four patients experienced a significant increase in dose to the brain stem, hippocampus and/or optic apparatus, which could have been prevented if the delivered plan had been adapted. The team note that none of the patients who had re-optimized plans experienced local or distant failures. Additionally, none experienced grade 3 or higher toxicities or developed radiation necrosis.
“Of the 27% of patients who demonstrated anatomic change during proton therapy, more than 50% had suboptimal delivered plans,” write the authors. “Re-optimization of these plans resulted in an improvement of CTV coverage up to 16% and a decrease in OAR dose up to 46%.” Because most changes were identified during the second or third week of a six-week course of treatment, the re-optimization effect would have been significant.
“Certain tumour histologies and locations might derive more benefit than others from this type of approach. Specific patterns of anatomic change, unrelated to tumour or patient characteristics, might help in selecting patients for MRI-based adaptive therapy,” the researchers write.
“We are planning to conduct a prospective pilot study that will specify timing and frequency of on-treatment MRI for different disease categories,” Acharya tells Physics World. “Through this study, we seek to understand the number of patients we need to image in order to detect one plan deviation.”
Intensity-modulated protons reduce side effects of whole-brain radiotherapy
Acharya is also the principal investigator of an accruing phase II study (NCT04065776) investigating the feasibility of reducing radiation doses to the hippocampi by using proton therapy for midline or suprasellar low-grade gliomas. Children enrolled in this study also undergo a weekly MRI scan, and treatment plans are adapted to shrinking tumour volumes if there is more than a 20% change in GTV. All children are also followed with longitudinal neurocognitive testing.
“This study might provide some insight on whether adapting to a smaller target volume can spare critical regions of the brain, such as the hippocampus, for children with midline or suprasellar low-grade gliomas, and whether sparing such regions will preserve neurocognition,” explains Acharya.



