
A key objective of radiotherapy is to increase the therapeutic ratio – by maximizing the death of cancer cells while minimizing radiation dose to normal tissues. One approach is to use high-atomic-number nanoparticles to enhance the efficacy of the incident radiation. This works because photons interacting with such nanoparticles produce photoelectrons and Auger electrons, thereby amplifying the local radiation dose and upping the therapeutic ratio.
Bismuth-based nanoparticles, which benefit from high X-ray absorption coefficient, low toxicity and low cost, hold promise as a potential radiosensitizer. Now a research team at Tsinghua University has investigated the use of bismuth gadolinium oxide (BiGdO3) nanoparticles as a new theranostic agent for enhancing radiation therapy, as well as acting as an imaging agent (Phys. Med. Biol. 10.1088/1361-6560/ab2154).
“Bismuth and gadolinium with high atomic number have shown the ability to enhance radiation,” explains first author Azimeh Rajaee. “Moreover, they both provide CT contrast, while gadolinium can be employed for MRI T1 contrast.”
Rajaee and colleagues used a simple, inexpensive sol-gel method to synthesize BiGdO3 nanoparticles with an average diameter of 11.3±1.6 nm. For in vitro and in vivo experiments, they modified the surface with PEG to reduce the toxicity and increase blood circulation time. The researchers first assessed biocompatibility by exposing MCF-7 breast cancer cells to nanoparticle concentrations from 0 to 500 µg/ml. They saw negligible change in cell viability compared with a control group, demonstrating the nanoparticles’ low cytotoxicity.
To evaluate the radiosensitizing effect of the BiGdO3 nanoparticles, the team performed a clonogenic assay on two breast cancer cell lines. The survival rate for MCF-7 cells irradiated at 2 Gy was 87%. Survival decreased to 70% and 49%, for irradiation plus 50 and 100 μg/ml BiGdO3 nanoparticles, respectively.
For 4T1 breast cancer cells, the survival rate decreased from 70% (radiation only) to 53% (radiation plus 50 µg/ml) and 42% (radiation plus 100 µg/ml). Irradiation at 4, 6 or 8 Gy showed similar patterns. For the 100 μg/ml concentration, this represents a sensitizer enhancement ratio of 1.75 and 1.66, for MCF-7 and 4T1 cells, respectively.
The researchers also used gel dosimetry to measure the dose enhancement factor (DEF). Comparing dosimetry results in the presence and absence of (500 µg/ml) nanoparticles revealed a DEF of 2.3.
In vivo investigations
Having proved the dose enhancement effect in vitro and using gel dosimetry, the researchers moved on to in vivo radiotherapy studies. They injected mice with 4T1 cells and divided the animals into four groups to receive: no treatment; intra-tumour injection of BiGdO3-PEG nanoparticles; X-ray irradiation at 10 Gy; nanoparticle injection plus 10 Gy irradiation.
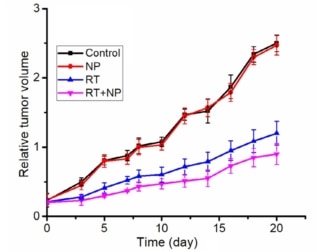
In the control group, tumour volume steadily increased, growing by about 10 times after 20 d. Irradiating the mice slowed this tumour growth, with tumour volume increasing by about 6 times and 4.5 times after 20 d, without and with BiGdO3-PEG nanoparticles. These results confirmed the radiosensitizing effect of the nanoparticles.
Finally, the team evaluated the use of BiGdO3 nanoparticles as a dual-modal imaging agent for CT and MRI. Comparing T1 MR images of mice tumours before and after intra-tumour nanoparticle injection showed that the nanoparticles increased the signal intensity and brightness. Likewise, CT images of mice demonstrated increased CT values after nanoparticle injection, confirming contrast enhancement.
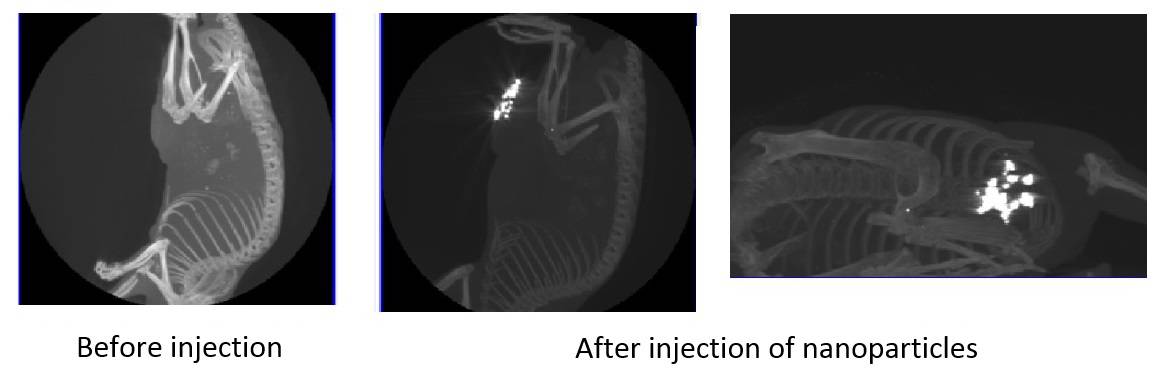
Following these findings, the researchers propose that the nanoparticles might be appropriate for improving image-guided radiation therapy, to not only improve CT and MR images, but also to increase the local radiation dose. “Our results show that the nanoparticles deserve further study in radiation therapy,” says Rajaee.
The researchers note that for this proof-of-concept investigation they injected the nanoparticles directly into the tumour. “However, for investigation of BiGdO3 distribution in tissues and organs, the nanoparticles should be injected intravenously,” Rajaee tells Physics World. “So further research needs to be done on surface modification of the nanoparticles with neutral polymers, and optimizing the size to enhance absorption into tumours according to the enhanced permeability and retention effect.”



