
Surgical removal of a tumour is one of the most common treatments for cancer. To help distinguish tumours from healthy tissue during excision, surgeons use fluorescence-guided surgery (FGS) to improve the visibility of structures that may not be seen clearly in the white light of an operating room. A London-based research team has now shown that multispectral short-wave infrared (SWIR) imaging combined with machine learning shows promise to improve the accuracy of FGS.
FGS uses tumour-targeted imaging agents to provide real-time visualization of tumours with molecular specificity and high-contrast margin delineation. The target tissue is stained with a near-infrared-emitting dye that preferentially binds to the surface of tumour cells. Most FGS set-ups use the absolute intensity of the infrared emission to discern which image pixels correspond to tumours. This intensity, however, is sensitive to the lighting conditions in the operating room, the camera setup, the amount of dye used and the time elapsed after staining. As such, intensity-based classification is prone to inaccuracies.

Writing in the Journal of Biomedical Optics, the team explains that by capturing multispectral SWIR images of the dyed tissue, and using machine learning to classify pixels based on their spectral characteristics, rather than intensity, more robust delineation of tumour tissue during FGS is possible.
Led by Dale Waterhouse of University College London, researchers from the UCL Great Ormond Street Institute of Child Health and the Great Ormond Street Hospital for Children NHS Foundation Trust created a multispectral SWIR fluorescence imaging system. The system illuminates tissue with a 785 nm fibre-coupled laser and uses a highly sensitive InGaAs camera coupled to a SWIR lens to collect the fluorescence emission. This fluorescence light is sequentially filtered through six long-pass filters, with cut-off wavelengths of 850, 950, 1050, 1150, 1250 and 1350 nm.
The researchers assessed their system using an animal model of an aggressive type of neuroblastoma, following injection of a neuroblastoma-specific fluorescent probe. They sequentially placed each filter in front of the SWIR optical system and captured six images, using these to construct image cubes (640 × 512 pixels × six filters) representing the fluorescence collected from 850 to 1450 nm.
Next, the team trained seven machine learning-based models to classify each pixel as tumour, non-tumour tissue or background, based on their spectra. The researchers also compared different normalization approaches designed to make pixel classification independent of the absolute intensity.
Using AUC=1 spectra normalization, the best performing model achieved a per-pixel classification accuracy of 97.5% (97.1%, 93.5% and 99.2% for tumour, non-tumour tissue and background, respectively). The team note that the normalization strategy made the results of the model far more robust to changes in imaging conditions.
Looking to the future
Waterhouse and collaborators advise that a second-generation system will require another method of multispectral imaging with higher temporal resolution, because image cube acquisition using a manual filter wheel was slow. They also recommend a system with higher spectral resolution or optimized spectral filter sets designed to better distinguish subtle differences between tumour and non-tumour spectra.
To apply SWIR imaging in the clinic, short exposure times are needed to enable video-rate imaging. Future work is required to optimize illumination, field-of-view, lenses and filters for an intraoperative SWIR platform. Additionally, a more precise means to draw regions-of-interest will be needed.
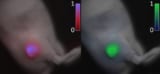
Delayed fluorescence imaging helps identify cancerous tissue during surgery
The researchers are optimistic. “If these results are validated in a first-in-human pilot study, multispectral SWIR fluorescence imaging could be incorporated into clinical practice to improve FGS,” they write. “With further development, multispectral SWIR FGS has the potential to revolutionize surgery. The eminent arrival of dozens of new imaging agents provides a timely opportunity for this technology. By enhancing the performance of these agents, multispectral SWIR FGS is poised to be instrumental to the advancement of FGS into the next generation.”
“Paediatric surgical oncology faces an ever-increasing need for novel technologies and devices that can help visualize tumours intraoperatively,” adds co-author Laura Privitera. “By using targeted fluorescence-guided surgery, we demonstrate the possibility of safely and specifically delineating tumour margins, allowing its differentiation from surrounding healthy tissue. Fluorescence-guided surgery is a game-changing innovation that will help surgeons to obtain safer and more complete resection.”



