Researchers in the US have discovered a natural mechanism whereby byproducts of radioactive decay are stored underground – rather than escaping into the wider environment. Evan Groopman of the US Naval Research Laboratory and colleagues studied an unusual sample of uranium ore that was mined from a rare underground geologic formation in the Oklo region of Gabon. The formation once functioned as a natural nuclear reactor, which sustained the same fission reactions as human-built nuclear power plants
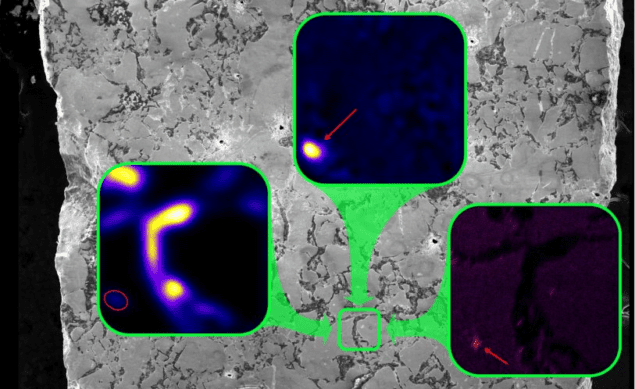
By analyzing the sample’s chemistry, they found that fission-produced caesium and barium had been captured in rock grains containing ruthenium – which is also a fission product. This natural process could be adapted into new storage technologies for waste from nuclear reactors.
The Oklo formation consists of 16 distinct sites and was discovered in 1972 when a worker at a French nuclear processing plant noticed a sample of uranium ore with a low relative abundance of uranium-235. Such a low abundance is usually found in depleted uranium that has been used as fuel in a nuclear reactor.
Neutron moderation
Researchers deduced that the uranium-235 had decayed via a fission chain reaction that occured about two billion years ago. They also figured out that the process was only possible because the uranium deposit was relatively large and flush with groundwater. Water acts as a neutron moderator, reducing the energy of neutrons emitted during fission and increasing the likelihood that they will go on to cause more fission events.
It takes an incredible confluence of conditions for these natural nuclear reactors to occur
Evan Groopman
The site studied by Groopman’s team sustained fission sporadically over about 24,000 years. “It takes an incredible confluence of conditions for these natural nuclear reactors to occur,” he says.
The ore sample was a small square tile about 4 mm wide and 1 mm thick. The researchers analysed it using a new instrument built by the team and called NAUTILUS. It can characterize a sample’s chemistry without having to dissolve it. The machine combines two different techniques, secondary ion mass spectrometry (SIMS) and accelerator mass spectrometry (AMS). “We made a Frankenstein of sorts,” says Groopman.
Both techniques identify the chemical makeup of a sample by ionizing atoms on its surface and then sorting them by mass. The SIMS can provide spatial information on where on the sample the ions originated, whereas AMS can detect trace elements.
Splitting molecules
NAUTILUS can spatially resolve grains down to 10 micron in size. In addition, the machine can take an extra step to split a molecule into constituent atoms. Many mass spectrometry machines only sort particles by mass and cannot necessarily distinguish between a heavy atom and a molecule of equal mass.
The ancient fission process produced several elements including barium, caesium, and ruthenium. Groopman and colleagues found that the barium and caesium would then bind to the ruthenium. Although the caesium has since decayed and thus disappeared from the sample, the team measured the daughter isotopes produced by the decay.
The binding of caesium is particularly significant, says Groopman, because the element is volatile and it usually escapes into the atmosphere in human-built reactors. In addition, caesium-135 has a half-life of two million years, which means that it remains risky as a nuclear waste product for a very long time.
The team also found that the sample has the lowest relative abundance of uranium-235 of any rock ever analyzed, to their knowledge. Normally on Earth, uranium-235 makes up about 0.7255% of uranium isotopes in a sample – whereas the Oklo sample contained about half that amount.
Remarkable precision
Also, by tracking the different ratios of barium and caesium isotopes, the team could tell that it took about five years for the ruthenium grains to form after fission in the reactor stopped. This precision is particularly remarkable because the absolute age of the uranium deposit can only be determined on the order of millions of years, says Rodney Ewing of Stanford University in the US, who was not involved with the work.

Are the laws of nature changing with time?
To design better nuclear waste storage, researchers have been drawing inspiration from these natural nuclear reactors for years, says Ewing. Groopman thinks that these ruthenium compounds might be especially useful. “Perhaps nuclear fuel storage casks could be lined with an additional mineral or element that binds with and captures the caesium,” he says. “Then the caesium can be stored over a longer term, so it has time to decay.” To that end, Groopman says that they need to further study the structure of the ruthenium metal sulfide compounds.
This further study could also help them better understand the natural nuclear reactors. “The overarching goal is to figure out how all these different [fission-produced] isotopes migrate and were contained in the reactor fuel,” says Groopman.
The research is described in the Proceedings of the National Academy of Sciences.



