Two new processes for producing different types of graphyne – a 2D allotrope of carbon that includes triple bonds – have been reported in independent papers. One paper – from researchers in the US and China – reports the first experimental synthesis of a bulk crystal of the most stable form of graphyne, which could potentially have multiple uses. The second – from researchers in South Korea – describes the discovery and synthesis of a hitherto unpredicted “holey graphyne”. However, some scientists are not convinced of the existence of this second type of graphyne.
Carbon is known for its ability to form numerous different allotropes, such as graphite, diamond and fullerene, by bonding together in different configurations. Graphite, for example, comprises 2D layers of carbon atoms held together by van der Waals forces, whereas diamonds consist of a 3D cubic lattice. Graphene is essentially a single layer of the carbon atoms that comprise graphite. Predictions about graphene’s properties date back to 1962, and when it was first exfoliated in 2004 it was confirmed to have remarkable strength, electronic mobility, flexibility and other qualities.
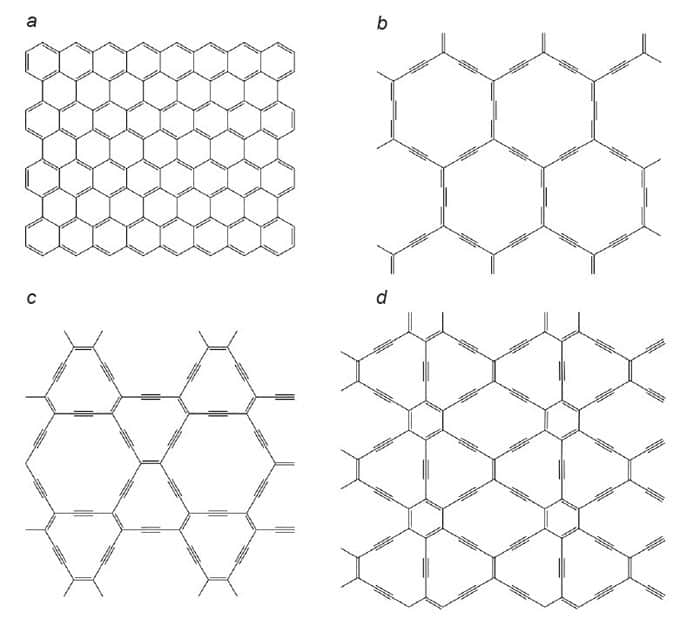
Researchers first predicted that graphynes could be stable back in 1987. Unlike graphene, there are several potential graphyne structures depending on the proportion of triple bonds and how they are distributed around the lattice. Various researchers have suggested graphynes could have remarkable properties of their own, such as highly directional electrical conductivity or ion mobility – which is extremely important for battery electrodes.
Bottom-up approach
However, it has previously proved impossible to produce a bulk sample of any graphyne. The mechanical exfoliation technique used to produce the first samples of graphene from graphite is impracticable, as no 3D material contains layers of graphyne. Instead, a bottom-up approach is needed to synthesize the material from precursor molecules.
Several researchers have proposed synthesis protocols for γ-graphyne – predicted to be the most stable isomer. In 1997, for example, materials chemist Michael Haley of the University of Oregon in the US proposed that γ-graphyne could be produced through alkyne metathesis. This is a coupling reaction between phenyl alkynes, squeezing out a small “co-product” and leaving aromatic rings connected by triple bonds. The problem is that defects inevitably form: “The co-product that you normally get from a typical alkyne metathesis is 2-butyne, which is a gas that bubbles out of solution and goes away,” explains Haley. “Well if you’ve got an error, how the hell do you correct for that error if the other piece has gone?”
Scientists led by Wei Zhang of the University of Colorado in the US and Yingjie Zhao of Qingdao University of Science and Technology in China solved this problem by adding a larger, less-volatile substitute to the reaction mixture that can also break the triple bond. It can break any triple bond, defective or not, but the defective bonds have higher energy, so these are more unstable. Moreover, says Zhang, “when the higher-energy bonds break open, they are more likely to form lower energy bonds”.
Making and breaking bonds
By allowing both bond making and bond breaking, therefore, the researchers drove the reaction towards the thermodynamically favoured product – perfectly crystalline γ-graphyne. The researchers say that, to the best of their knowledge, they have produced the first demonstration of any graphyne with long-range crystalline order, although other groups have previously reported tiny fragments of carbon that contained triple bonds.
Haley is impressed with the team’s paper, which is published in Nature Synthesis. He believes the door is now open to find out what the material is useful for. “There have been all of these predictions: it’s going to be an exceptionally strong material; it’s going to be great for batteries because you’ll be able to move lithium ions through it – who knows?” he says. “Anybody anywhere should be able to replicate what they’ve reported, and that to me is the strength now: you finally have the material in more than sub-milligram quantities, and you can go and begin to fully investigate all these predicted properties.”
Jeffrey Moore of the University of Illinois Urbana-Champaign in the US agrees and sees two obvious follow-up studies: “One is to understand how this perfect or near-perfect structure is being made by the interplay of kinetics and thermodynamics – there’s some deep mechanistic questions that, if understood, would allow us to make more similar kinds of materials like this more predictably,” he says; “The second is to do chemical modifications, where you introduce defects deliberately to create structures that bring new function.”
Unexpected structure
The second graphyne paper is published in Matter and reports a previously unexpected graphyne structure that comprises networks of benzene rings connected by strained, triple-bonded eight-membered rings. A molecule comprising two linked benzene rings was first reported in 1974, and the researchers decided to investigate whether polymers comprising multiple linkages – creating ring-shaped networks of benzene rings with nanometre-sized pores – could be stable.
Computational modelling established that it could be stable, says Hyoyoung Lee of Sungkyunkwan University in South Korea, so his team set out to develop a synthesis protocol. “We made the intermediate from six steps of organic synthesis, and then started from that,” he says. Spectroscopic analysis suggests that the material has an electronic bandgap of 1.1 eV, say the researchers: “We are going to use this material for sensors, or if possible for photodetectors, and also as a channel material for a thin-film transistor,” says Lee.
Could graphynes be better than graphene?
Some researchers, however, are sceptical that the material even exists: “Everything in the literature to date suggests this should not do what the authors are claiming it does,” says Haley; “Whereas the description of how they made the monomer is beautifully detailed, the description of how they made the polymer has essentially no detail.”
Lee says, “People are trying to understand ‘How can you make this?’ – That’s still something of a black box…But the bottom line is we made it. We have the simulations, and we have the transmission electron microscopy. Our molecular structure conforms with the spectroscopic measurements”. The researchers also support their claim using several other imaging techniques.
Haley, however, is cautious: “I’m finding now that we can take lots of pictures through STM, AFM, whatever form of microscopy you want, but the devil in the details is not there as it should be,” he says; “Is it what they claim it to be? I remain to be convinced.”