Chemists at the Technical University of Munich have used scanning tunnelling microscopy (STM) to give the world’s first insight into the atomic behaviour of catalytically active sites during electrochemical reactions. The results were obtained via a little-used variant of the technique which measures noise in the signal. The information can be used to improve reactions and processes in many fields through the targeted design of better catalysts.
A staple analysis tool in the field of surface science, STM involves bringing an atomically sharp metallic probe, or tip, very close to a conducting sample. When a bias is applied across the two, electrons that were originally localized on the tip are given the energy to “tunnel” across the empty space onto the sample. The flow of tunnelling electrons gives rise to a current, which is monitored to build up information about the surface of the sample.
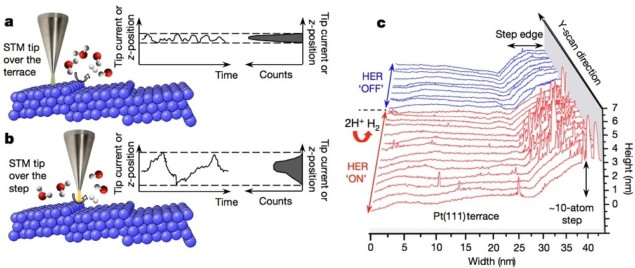
Writing in Nature, Aliaksandr Bandarenka and colleagues report how a modified version of this technique – scanning noise microscopy (SNM) – can be used to investigate the link between the atomic structure of a catalyst and its chemical activity during a reaction.
Listen closely
As with STM, SNM relies on the idea that the quantum-mechanical tunnelling barrier is altered by the structures and processes beneath the device’s tip. The researchers found that sites of catalysed chemical reactions produce a higher level of noise in the tunnelling-current signal observed under a constant bias. This noise manifests as a broader distribution of tunnelling-current magnitudes received from the active area, and can be picked out through statistical analysis.
Investigating these active sites does not require any modification to the electrochemical STM setups commonly found in many labs, and can proceed directly under reaction conditions. Although not investigated in the reported research, there is also scope to improve upon the technique to include chemical sensitivity in the measurements.
Predictions confirmed
Using SNM, the Munich group studied the catalytically active sites of gold and palladium surfaces during the economically important hydrogen-evolution and oxygen-reduction reactions, and found them exactly as predicted by theoretical calculations. The relationship between the atomic structure of a catalyst and its chemical activity during a reaction has long been suspected within the field, and hence this proof has been highly anticipated.
The unprecedented resolution afforded by STM in the 1980s allowed the electronic and topographic construction of materials to be studied at an atomic level, which was vital for the understanding of a huge variety of chemical and physical interactions. The work by Bandarenka’s team can be expected to do the same for catalysis, with knock-on effects reaching much further afield.
Heterogeneous catalysis, in which the phase of the catalyst is different to the phase of the reactants, underpins 80% of the world’s chemical and energy production processes, and provides everything from fertilizer for food crops, to hydrogen for use as an alternative source of energy. A better understanding of the dynamics will allow the design of new and effective industrial catalysts, leading to more efficient reactions and a cleaner, greener and less energy-consumptive future.
Full details of the research are reported in Nature 10.1038/nature23661



