
The ability to quantify and localize spatial variations in lung function could help doctors more effectively diagnose, monitor and treat many respiratory diseases. A multidisciplinary team of researchers in Australia has developed a novel tool for measuring regional lung function and used it to detect and characterize a disease in mice similar to cystic fibrosis (CF).
CF is a hereditary disease that causes the body to make abnormally thick and sticky mucus. This mucus can hinder breathing and lead to severe lung infections and permanent damage. CF progressively reduces quality-of-life and ultimately leads to premature death. Lung damage caused by CF is often heterogeneous, or non-uniform, so local treatments targeting patches of damaged tissue can slow the progression of the disease to preserve quality-of-life and lengthen overall survival.
The best method for assessing lung function consists of measuring how much air a patient can inhale and exhale, in addition to how quickly this volume of air is exhaled – a technique called spirometry. This information is then used to identify breathing patterns commonly associated with respiratory conditions, like CF. Critically, spirometry only assesses the global health of the entire lung and cannot localize subtle, non-uniform deficiencies.
Recently, Freda Werdiger from Monash University’s Department of Mechanical and Aerospace Engineering led a team of biomedical engineers, physicists, mechanical engineers and respiratory physicians from Adelaide Women’s and Children’s Hospital and the University of Adelaide to overcome these limitations. This collaborative team applied X-ray velocimetry (XV) to non-invasively generate high-definition and sensitive images of the real-time airflow through the lungs of mice.
X-ray velocimetry
XV is a combination of high-speed imaging and post-processing analysis that produces a detailed ventilation map of the lungs.
To achieve high-speed image acquisition, Werdiger and her team utilized propagation-based phase-contrast X-ray imaging (PCXI). Unlike conventional radiography, which relies solely on X-ray absorption, PCXI additionally leverages the diffraction of X-rays at material interfaces.
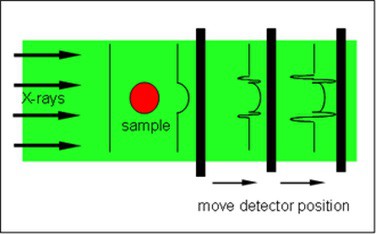
By placing the detector further from the sample than is typical for radiography, diffracted X-rays can interfere with the un-diffracted X-rays. This produces an interference pattern that highlights structural boundaries. This results in enhanced image contrast and enables high-resolution images to be acquired in a brief time.
When combined with tomography methods, PCXI can rapidly generate detailed, three-dimensional images of fine lung structures.
The post-processing analysis for XV involved the multiple tomographic PCXI images acquired throughout the breathing cycle. The researchers used a computational method known as particle image velocimetry to calculate displacement vectors between consecutive images and determine the three-dimensional speed and direction of lung motion throughout the breathing cycle. This provided an entirely non-invasive way to calculate the volume of air that flows through the individual airways of the lungs.
Characterization of CF-like disease in mice
The researchers tested the feasibility of using XV to acquire reliable and quantitative measures of respiratory function in a cohort of mice with CF-like lung disease and their healthy littermates. Their results were recently published in Scientific Reports.
First, they generated maps of regional lung expansion for both healthy and diseased mice. These maps clearly showed the presence and location of confined areas of airflow deficits in the diseased animals, a characteristic of the patchy nature of CF.
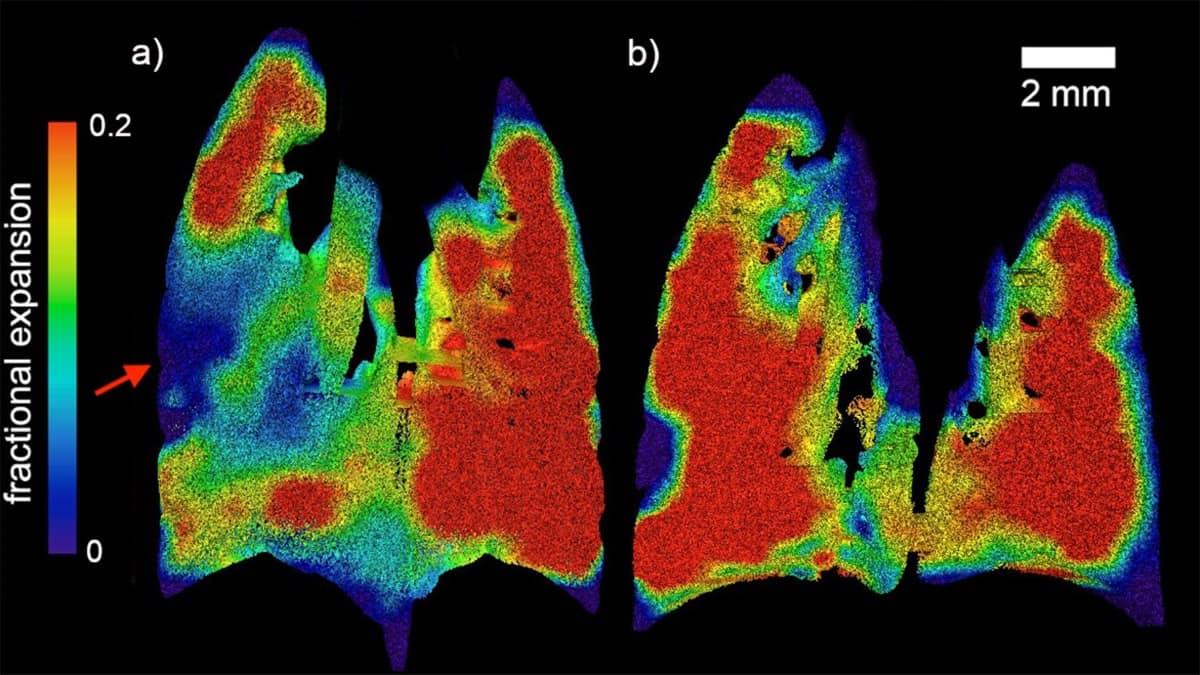
Next, the researchers developed a method to quantify the distribution of disease in individual mice. Their novel quantification correlated well with measurements by the conventional forced oscillation technique, while offering a higher level of specificity.
“This study shows how researchers from many different backgrounds can come together in collaboration and work together to improve the lives of people living with this terrible disease,” says Werdiger. “I hope to see XV being used to diagnose and monitor many different diseases,” she added, a wish that is likely to be realised. With its recent commercialization by Andreas Fouras of 4DMedical, this technology is well positioned to assist in global research and clinical care that could improve the length and quality-of-lives of people with CF and other respiratory diseases.



