Adding optoacoustic (OA) imaging to ultrasound (US) could improve the diagnosis of breast cancer, according to findings from a multidisciplinary research team in Cambridge, UK. The combination enables visualization of functional blood vasculature overlaid with structural features of the breast.
To help accelerate the clinical application of this combined technique, the team developed a simple feature set using single-wavelength OA data from an integrated OA-US imaging system that can identify malignant breast lesions based on their vascular patterns. The researchers describe their findings in Photoacoustics.
A low-cost OA-US device using this feature set could increase the number of early breast cancer diagnoses, especially in women living in low-income countries, where breast cancer survival rates are less than 40% (compared with 80% in high-income countries). The proposed device could also expand breast cancer screening in populations with limited access to mammography.
Ultrasound imaging alone tends to have low sensitivity for breast cancer detection, and cannot always differentiate between benign and malignant lesions. OA imaging – a potentially low-cost technique based on optical excitation and acoustic detection – is being evaluated in clinical studies for breast cancer diagnosis, but the current analysis process is quite complex.

Principal investigator Sarah Bohndiek, of the University of Cambridge’s Cancer Research UK Cambridge Institute and department of physics, explains that the researchers’ objective was to simplify acquisition of OA-US data and create a simple imaging feature set that was easy to learn and clinically feasible to implement.
The team generated the feature set using images from 96 breast lesions in 94 patients with benign, indeterminate or suspicious breast abnormalities at Cambridge University Hospitals NHS Foundation Trust. The first 38 lesions (including 14 malignant and eight benign) were used to develop the feature set; the others were used for validation.
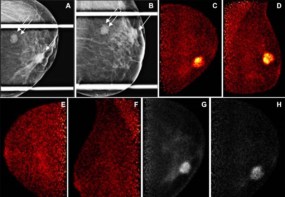
All patients in the study underwent mammography, breast ultrasound and OA imaging – performed using an OA device that also incorporated low-frequency tomographic ultrasound. The researchers used an excitation wavelength of 800 nm, which minimizes absorption by water and lipids, to create images showing the morphology of blood vessels surrounding a solid breast lesion. The use of a single wavelength simplified OA image processing and visualization, offering the possibility of future system simplification and cost reduction.
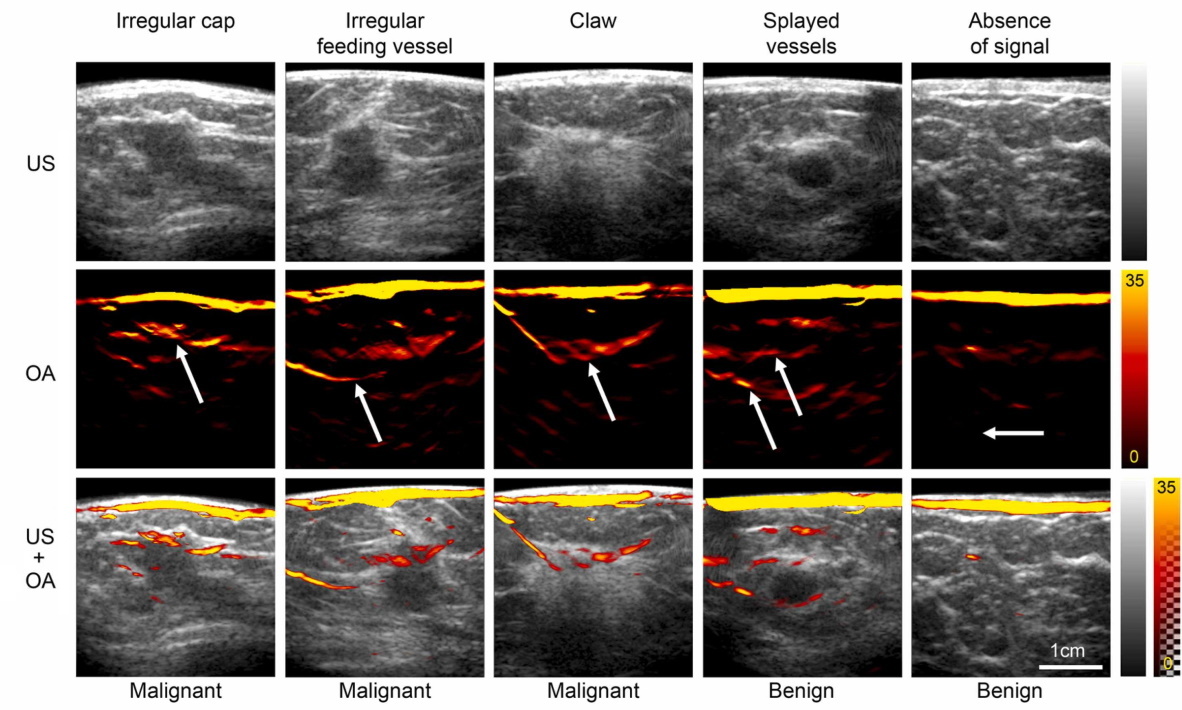
The researchers analysed the OA and US images separately and in combination, looking for patterns of blood vessels representative of healthy breast tissue, benign disease and malignancy. Benign lesions demonstrated no vascularity or vessels that were draped over the lesion without penetrating it. Malignant lesions had irregular feeding vessels that penetrated into the lesion and/or a disorganized irregular pattern around them. The internal appearance of the lesions did not differentiate benign and malignant lesions and was not used.
The researchers selected three features of malignancy that would upgrade any solid lesion to a BI-RADS 5 classification (highly suggestive of malignancy): irregular cap, irregular feeding vessel and claw sign. The presence of benign features – no vessels or vessels splayed over the lesion vessel – would downgrade a lesion to BI-RADS 2 (benign).
Two breast radiologists validated the feature set by independently interpreting the OA-US validation images (31 malignant and 13 benign solid lesions). It took only 20 min of training for them to become proficient in using the feature set. They were asked to use the features to classify lesions by BI-RADS category, as well as to classify the patients’ diagnostic ultrasound exams and mammography images.
The breast radiologists interpreted the OA-US images with a sensitivity of 96.8% and a specificity of 84.6%, with one false negative and two false positives for each reader. In comparison, mammography yielded three false negatives and two false positives for each reader, and ultrasound generated one false negative and six and seven false positives. Importantly, all of the mammography and ultrasound false negatives were correctly identified as positive by OA.
Deep learning accelerates super-resolution photoacoustic imaging
Bohndiek points out that OA-US requires practical experience to optimize the standard operating procedure and obtain high-quality image data, and for this reason, future multicentre validation studies should consider operator dependence and independent calibration.
“We have been undertaking validation studies of the OA-US device in the context of developing stable test objects (phantoms) that can be used by medical physicists for QA/QC once the devices are used routinely in the clinic,” she tells Physics World. “We are also planning to apply the system in the future to monitoring of response to radiotherapy treatment in breast cancer.”