Immunotherapy with checkpoint inhibitors is becoming an increasingly important tool in the treatment of several cancers. These checkpoint inhibitors, which block the proteins that stop the immune system from attacking cancer, can reactivate immune cells to enhance tumour killing. However, only a subset of patients respond.
A key determinant of successful checkpoint blockade therapy is the presence of CD8+ T cells, which play a central role in anti-tumour immune responses. As such, visualizing CD8+ T cells in vivo before and during treatment could provide insight into the mechanisms of immunotherapy and potentially predict a patient’s treatment response.
In a clinical trial sponsored by LA-based biotech company ImaginAb, a research team headed up at Memorial Sloan Kettering Cancer Center has now performed the first-in-human imaging of a tracer designed to non-invasively visualize the immune system (J. Nucl. Med. 10.2967/jnumed.119.229781).
The researchers used 89Zr-IAB22M2C, a minibody (antibody fragment) designed to target CD8+ T cells and radiolabelled for PET imaging. They tested the tracer, produced by ImaginAb, in a dose-escalation study of six patients with solid tumours (melanoma, lung cancer or hepatocellular carcinoma) who were undergoing or likely to receive immunotherapy.
Patients were injected with approximately 110 MBq of 89Zr-IAB22M2C, at minibody mass doses of 0.2 up to 10 mg. The researchers then performed whole-body PET/CT at various post-injection time points. They note that the infusion was well tolerated with no immediate or delayed side-effects observed for any mass dose.
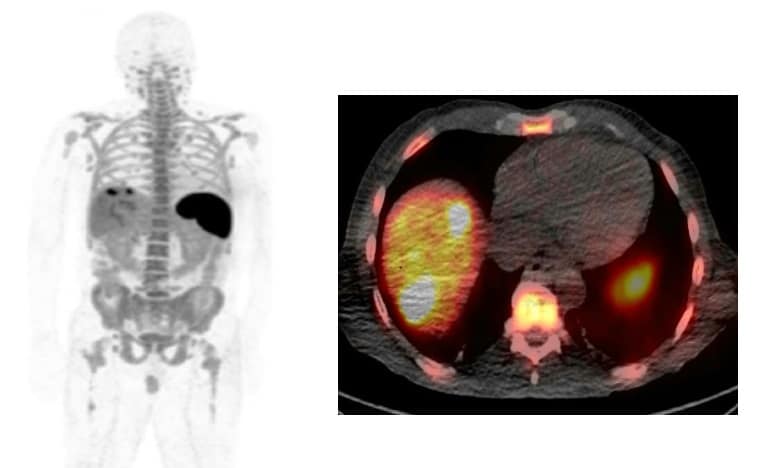
Evaluating the biodistribution of 89Zr-IAB22M2C showed that the minibody targeted CD8+ T cell-enriched tissues, with high uptake seen in the spleen, bone marrow and lymph nodes. The biodistribution was dependent on the administered minibody mass, with uptake almost completely confined to the spleen at the lowest mass dose (0.2–0.5 mg).
The highest uptake was always seen in the spleen, followed by the bone marrow. As the mass dose increased, however, blood pool retention increased and higher liver uptake was seen at later times. The team notes that this may be a saturation effect, due to competitive binding from the increasing amount of cold (non-radiolabelled) minibody.
The researchers also took multiple blood samples from the patients to assess serum clearance. Clearance was typically bi-exponential and also depended upon minibody mass, with rapid extraction of lower minibody masses from circulation and slower serum clearance for higher masses (5–10 mg).
Tumour lesions showed variable 89Zr-IAB22M2C uptake among patients, possibly due to differing treatment profiles or the variable presence of CD8+ T cells. In two patients, PET revealed prominent uptake in lesions imaged at lower masses. Both patients were receiving immunotherapy and may thus have had a higher concentration of CD8+ T cells. Conversely, three patients with metastatic lung cancer did not show prominent uptake, possibly related to a lack of ongoing treatment with immunotherapy and therefore lower levels of T cells.
The researchers conclude that PET with 89Zr-IAB22M2C is safe and feasible, and that the minibody successfully targets CD8+ T cell-rich tissues. Initial results suggest favourable kinetics for early imaging within 6–24 hr, with uptake seen in normal lymph nodes and tumour lesions as early as 2 hr post-injection.
Due to the small number of patients in the study, the researchers could not establish differences in lesion uptake among minibody mass doses. However, lower masses (less than 5 mg) seemed to provide a more favourable balance of normal tissue and lesion visualization.
“This novel imaging agent has the potential to non-invasively assess the presence of CD8 T cells in patients’ tumours and results of this initial assessment are encouraging,” says lead author Neeta Pandit-Taskar. “With more research, this technology may ultimately serve a critical role as a biomarker of immunotherapy outcome and inform clinical trials of novel immunotherapies that act mechanistically through the presence of CD8 T cells.”
The ImaginAb team is now performing further evaluations in more patients and a study incorporating parallel biopsies is also accruing patients.