Radiotherapy technologies have advanced significantly over the past couple of decades. The introduction of intensity-modulated irradiation and particle therapies have helped maximize the dose differential between tumours and normal tissues, while image guidance has reduced delivery uncertainties. One remaining hurdle to address is motion of the patient and tumour during treatment.
Conventional motion management approaches assume that radiation delivery is far slower than physiological motion. But recent preclinical studies have shown that FLASH radiotherapy – an ultra-rapid approach using dose rates exceeding 50 Gy/s – vastly reduces normal tissue toxicity without impacting therapeutic efficacy. And in addition to this potential radiobiological advantage, this high-speed irradiation effectively freezes motion.
FLASH has been demonstrated preclinically, but current clinical linacs are too slow to deliver FLASH to deep-seated tumour targets in humans, requiring a dose rate increase of at least 300 times. With this goal, US researchers are developing the PHASER platform for FLASH radiotherapy. They designed PHASER to be compact, power efficient, economical and clinically efficient – factors that will also help increase global access to radiotherapy (Radiother. Oncol. 10.1016/j.radonc.2019.05.005).
“PHASER is designed to fit inside a standard shipping container for rapid deployment, and to run on solar power and battery storage,” explains Billy W Loo Jr from Stanford University School of Medicine. “The 3D machining techniques used to create accelerator components greatly reduce the part count and thereby the manufacturing costs. The extremely rapid treatment combined with fast high-quality imaging also enable workflows that greatly increase patient throughput. All these features benefit advanced clinics, but also reduce access barriers in lower resource settings.”
Four core technologies
PHASER will provide near-instantaneous delivery of highly conformal image-guided radiotherapy. Loo and co-authors Peter Maxim and Sami Tantawi describe four core technical innovations that enabled its development.
First, they designed a compact, power-efficient linac called DRAGON that exploits a fundamentally new accelerator technology to generate hundreds of times the beam output of a conventional medical linac.
Production of high accelerating gradients (above 100 MeV/m) in linacs is usually limited by RF breakdown, caused by high surface magnetic fields that create localized pulsed heating. In their design, the researchers optimized the shape of the accelerating cells to minimize the peak surface magnetic fields. This reduces RF breakdown at high gradients and maximizes power efficiency by minimizing heating losses.
To install PHASER in an existing treatment vault, it must incorporate an extremely compact RF power source with a large duty factor. The researchers developed a 50 cm-high klystron that generates 330 kW peak RF power when operating at only 60 kV. The outputs of multiple “klystrinos” are combined via an RF phased-array power distribution network.
PHASER will incorporate 16 klystrinos and direct their summed power (5.3 MW peak) to any one of 16 stationary DRAGON linacs, providing 16-beam intensity-modulated FLASH radiotherapy. The system can switch beam direction in just 300 ns without requiring gantry rotation.
The researchers also created an all-electronic scanning pencil-beam high-speed intensity-modulated X-ray source (SPHINX) to replace the multileaf collimator (MLC) and eliminate mechanical motion. Each electron beam pulse is electromagnetically steered onto a stationary target to form a rapid scanning source of X-rays, which are collimated by an array of channels in a tungsten block.
Each channel forms a diverging beamlet and the spacing between beamlets ensures complete tumour coverage. For a sample lung-cancer case, a simulated SPHINX-based treatment plan demonstrated similar dose conformity as the clinically delivered MLC-based plan.
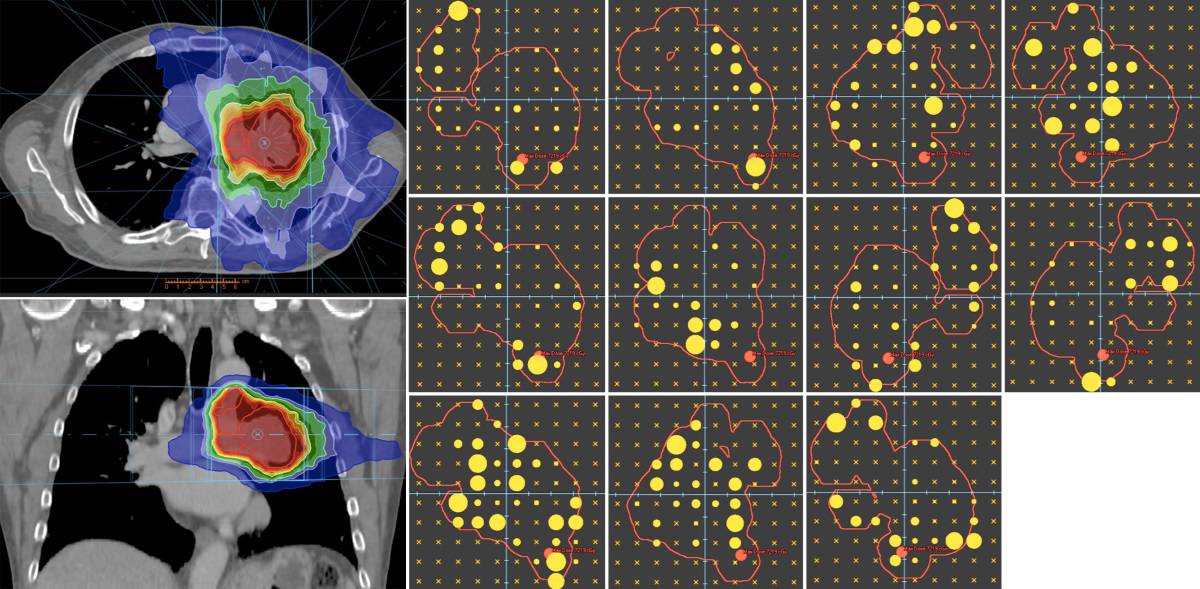
Ultra-rapid beam delivery also requires fast, high-quality image guidance. For this, the team integrated a multi-detector CT into the system, providing diagnostic-quality volumetric imaging at sub-second speed. A non-coplanar beamline geometry allowed the beams to share a common isocentre with a full-ring CT scanner for simultaneous imaging and treatment.
As well as providing position verification before and during treatment, this set-up enables an efficient adaptive planning workflow. Combining rapid imaging and delivery also creates new therapeutic possibilities, for example, temporally synchronizing irradiation with the intra-tumoural concentration peak of an injected systemic agent monitored via dynamic CT.
The team has prototyped all of these individual components, which are in various states of testing. “We are now finalizing the component designs to the specifications required for a clinical version of PHASER,” explains Loo. “We are also building a fully integrated single beamline, which will be a stepping stone to building multiple integrated beamlines and, eventually, the full 16-beamline version envisioned for clinical use.”
Loo notes that the single beamline will also be used for radiobiology research. “We are currently engaged in FLASH radiobiology research using modifications of existing accelerators, but the beamline based on PHASER technology will enable more advanced experiments,” he tells Physics World.