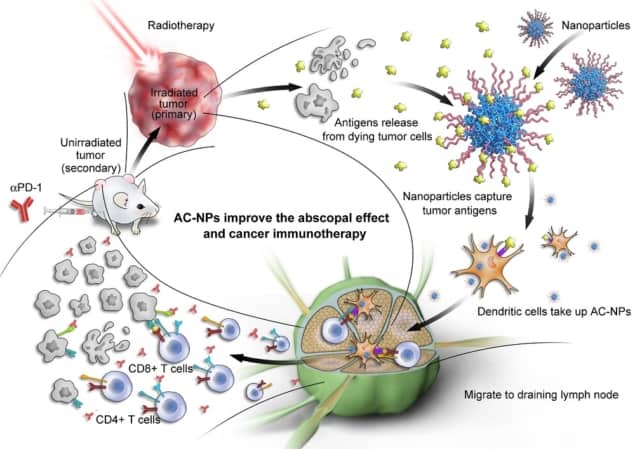
Scientists have improved cancer treatments in recent years by combining radiation therapy with immunotherapy. Now, researchers in the US and China have engineered nanoparticles to improve outcomes further. The nanoparticles capture tumour-derived proteins at the treatment site, providing a kick to the immune system and enabling it to detect and respond to cancer cells elsewhere in the body.
On its own, radiation therapy works by damaging cancer cells’ DNA, killing them and preventing them from propagating their defective genetic material. Immune cells arriving at the tumour site after irradiation use mutated proteins released by the dying cells to coach other immune cells into recognising and fighting cancer elsewhere. This phenomenon is known as the abscopal effect, and it results in tumour shrinkage even outside of the sites targeted with radiation therapy. Immunotherapy drugs known as checkpoint inhibitors are given alongside radiation therapy to help the body’s immune system marshal its attack on cancerous cells.
Writing in Nature Nanotechnology, a group comprising scientists at the University of North Carolina, Duke University Medical Center and Memorial Sloan-Kettering Cancer in the US, and Xuzhou Medical University in China, outline how antigen-capturing nanoparticles (AC-NPs) can enhance the abscopal effect by attaching themselves to tumour-derived protein antigens (TDPAs). The increase in immune response is thought to result from the size of the nanoparticles, which make the TDPAs much more attractive to the immune system. When TDPAs are bound to nanoparticles, the body mistakes them for something foreign such a virus, and responds more aggressively.

Starting with nanoparticles made from PGLA, a biocompatible and biodegradable polymer, the researchers prepared several formulations of AC-NPs by surface modification. Different coatings were attached to the AC-NPs through a number of mechanisms, and the group found that the surface chemistry determined the diversity and composition of the proteins captured.
The attachment of TDPAs to AC-NPs was shown to translate to a more robust activation of T-lymphocytes (T-cells), which play a central role in cell immunity. This increased therapeutic effect led to improved survival when tested in mice. The scientists found that 20% of the mice had a complete response rate when treated with the nanoparticles alongside radiation therapy, compared to none of those mice that received radiation therapy alone. Additionally, when the nanoparticle-treated mice were re-injected with cancerous cells three months after treatment, the mice rejected the cells, demonstrating that the treatment strategy outlined induces a durable response, increasing immunity to the tumours long-term.
Traditional methods of immunotherapy focus on administering one or several chosen antigens. The researchers believe that the limited success of this approach could be due to its failure to take into account diversity in tumour cells. The novel method outlined here is more promising as the immune system is exposed to a wide variety of TDPAs in a patient-specific manner. This offers opportunities for the future in precise and personalized medicines when treating patients suffering from extensive cancers.
Full details of the research are reported in Nature Nanotechnology.