Cancer stem cells (CSCs) are responsible for tumour growth and metastatic spread, as well as cancer cell repopulation following chemotherapy or irradiation. Finding safe and effective methods to destroy CSCs is of critical importance in cancer treatment, because they tend to be resistant to both radiation and chemotherapy.
The answer may lie in the use of microsecond pulsed electric fields (µsPEF), according to researchers from the Italian National Agency for New Technologies, Energy and Sustainable Economic Development (ENEA). Exposure to µsPEF provides an effective, destructive tool to retard tumour growth and when followed by radiation, could stop malignant tumour growth entirely. The researchers demonstrated the effectiveness of this approach on laboratory mice with medulloblastoma tumours, who experienced no tumour regrowth for nearly four months following the combined treatment.
Described in the International Journal of Radiation Oncology, Biology, Physics, this finding could have significant implications for improving cancer therapy, in particular, treatments of malignant brain tumours. In addition to improving survival outcomes, µsPEF radiosensitizes the CSCs prior to radiotherapy. This enables the delivery of lower doses of radiation to the brain, which could help minimize the risk of neurocognitive damage, particularly when treating paediatric patients.
Selective sensitization
Pulsed electric fields of high amplitude and short duration provide a powerful means to induce cell electroporation, in which the cell membrane becomes increasingly permeability to ions and macromolecules. One such approach, electrochemotherapy, which enables chemotherapy drugs to better permeate cell membranes, is now widely used to treat superficial and deep tumours. Others, such as irreversible electroporation (IRE) and high-frequency IRE (H-FIRE), cause direct, nonthermal cell death. The effectiveness of IRE and H-FIRE for treating a range of cancers is currently being evaluated in numerous clinical trials.
These electrically mediated therapies also offer the possibility to selectively target CSCs. CSCs are present in brain cancers and are believed to be responsible for their fast regrowth rate and the extremely high recurrence of brain tumours following treatment. Brain cancers in children are of particular interest, such as medulloblastoma, the most common malignant paediatric brain tumour.
Lead author Mirella Tanori and colleagues investigated the effects of µsPEF on D283Med cells, a human medulloblastoma cell line reported to be rich in CSCs, and on a normal human astrocyte (NHA) primary cell line.
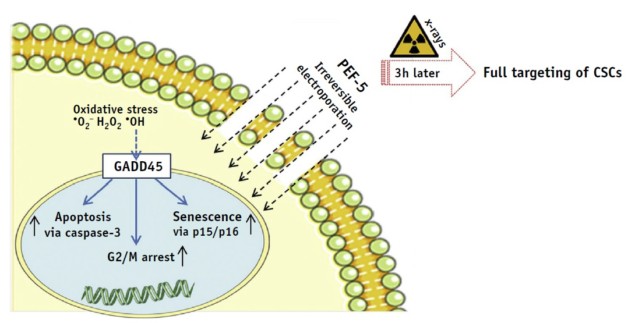
The researchers report that cell membrane permeability of D283 cells increased proportionately to the number of electric pulses administered. They identified a specific pulse protocol – five 40 µs pulses at 0.3 MV/m (PEF-5) – that produced high cell mortality within 72 hours. PEF-5 exposure reduced the clonogenic capacity (a cell’s ability to clone itself) of D283 cells by four times compared with sham-treated cells. The team note that NHA cells were resistant to this treatment, suggesting that NHAs have a higher threshold for irreversible electroporation than D283 cells.
Exposure of D283 cells to PEF-5, followed 3 hr later with 2, 5 or 8 Gy irradiation, demonstrated the effectiveness of the combined treatment. Notably, PEF-5 reduced clone formation with the same efficacy as the highest X-ray dose delivered, implying that PEF-5 could be used as a pre-treatment to de-escalate radiation dose.

Electric pulses act as stressors for cell membranes, causing exposed cells to generate reactive oxygen species to defend themselves. Thus the team also evaluated ROS production. In D283 cells, they observed a significant ROS increase 3 hr after the electric exposure. DHE cells, however, did not show this increase after PEF-5 exposure.
In vivo success
The team also investigated the use of µsPEF on medulloblastoma tumours in laboratory mice – and the results were similarly impressive. Compared with sham-treated mice, PEF-5 exposure alone inhibited tumour growth by 46.47%, while irradiation at 5 Gy inhibited growth by 87.18%, 43 days following treatment. The combination of PEF-5 and a lower radiation dose of 2 Gy inhibited tumour growth in mice by 100%, and there was no subsequent growth for up to 110 days.
“Pulsed electric field exposure may play an important role in sensitizing CSCs, also blocking their proliferation capacity and hence possibly promoting a stronger action with X-rays on the pre-treated D283 cells,” write the authors. They also believe that the combined treatment represents “an interesting therapeutic strategy to selectively target CSCs, safeguarding the healthy tissues and overcoming radiation therapy-associated cognitive disabilities typically associated with brain tumour therapies.”



