Nitric oxide (NO) plays a crucial role in many biological processes, including vasodilation, immunity and neurotransmission, but it is neurotoxic in excess. Detecting this gas is important for diagnosing inflammatory diseases such as asthma, where it is present in exhaled air, for example. NO also serves as a signalling molecule in carcinogenesis and the growth of some tumours, such as breast cancer.
NO is usually detected using techniques like chemiluminescence and amperometric measurements, but these have their drawbacks and limitations. Chemiluminescence only works for total gas volumes greater than a litre, for instance, and amperometry suffers from large cross-sensitivities to other small molecules such as carbon monoxide (CO).
Sensitivity of 10 ppm in a sample volume of just 10-4 litres
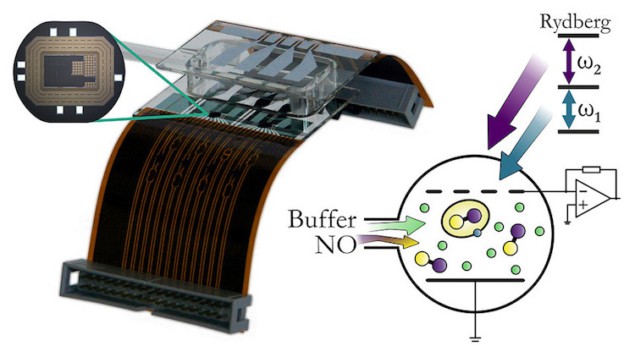
Researchers led by Harald Kübler of of the University of Stuttgart in Germany have now put forward a proof-of-concept for a new optogalvanic gas sensor for NO based on Rydberg excitations that they say could overcome the problems of currently employed methods. The device combines the advantages of optical and amperometric techniques, and a sensor prototype they made can detect NO concentrations of less than 10 parts per million (ppm) in sample volumes as small as 10-4 litres at atmospheric pressures and temperatures.
In their work, Kübler and colleagues used a pair of Nd:YAG pumped dye lasers to create Rydberg-excited NO molecules in a glass chamber. The chamber also contains helium (He) gas, two metal electrodes and a high bandwidth temperature-compensated transimpedance amplifier (TIA). The NO and He are prepared and stored in a stainless-steel vessel.
The Rydberg-excited NO molecules contain an electron excited to a higher state but the electron is only weakly bound, which means that it can be easily ionized by, for example, low-energy collisions in the chamber. The charges coming from the ionization of the excited molecules are detected as a current by the electrodes and this current is then converted into a voltage by the TIA. It is thus read out as a clear and unique indication of the presence of NO.
“Although the measured sensitivities in this proof-of principle-experiment are still orders of magnitude worse compared to other gas sensors such as chemiluminescence based ones, our results do indicate the potential of the proposed Rydberg detection scheme for making a sensitive and selective NO sensor that works at atmospheric pressures,” say the researchers.
The team, which includes scientists from the University of British Columbia in Canada, says that it now plans to construct a mixing apparatus that works in through-flow rather than in static mode and in which stainless steel parts are reduced to a minimum. This will allow all surfaces of the experimental chamber to be saturated with NO, so increasing the device’s sensitivity to the gas. Such a set up could possibly allow for practical applications such as NO detection in breath.
The sensor is detailed in Applied Physics Letters 10.1063/1.5024321.



