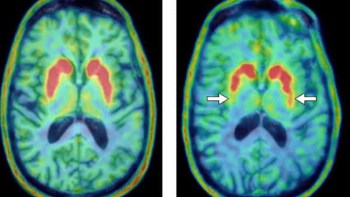
Cancerous cells have a higher metabolic activity than normal healthy cells, and clinicians use this characteristic to trace cancer metastasis within a patient’s body. 18F-flurodeoxyglucose (FDG), a glucose analogue, is a frequent candidate for these types of assessments as its distribution correlates to metabolic activity. This distribution can then be evaluated by capturing the gamma-ray signals emitted within a patient using PET. Static PET images enable a qualitative assessment of the distribution of the radiotracer, but to improve disease prediction, a more quantitative approach is required.
A kinetic analysis of radiotracer distribution over time would provide quantitative results. However, this requires ongoing measurement of radiotracer concentration in blood. A few methods for continuous radio-tracing exist, but there are logistical and technical issues that make them impractical for clinical use. For instance, one method requires continuous blood drawing from a patient, while another relies on non-specific input functions generated from population-based patient data.
Frustrated by existing tools, Charles Scarantino, a retired radiation oncologist, and Josh Knowland, an electrical engineer, co-founded Lucerno Dynamics to develop clinically useful devices based on scintillation detection of radiation. They describe a prototype device that they’ve developed and report on the initial feasibility tests in blood vessel mimics (J. Nucl. Med. Technol. 10.2967/jnmt.118.212266).
“Our motivation was to develop a simpler, patient-friendly method of measuring the concentration of radiotracer,” says Knowland. “Our approach would be personalized to the individual patient, would not require additional patient time in the PET scanner, and would not require sampling of blood for external handling and measurement.”
A simple system
The device is constructed from polystyrene-based scintillating fibres that convert radioactive energy into visible light. The fibres don’t have the power to distinguish gamma-ray radiation; instead they detect the beta particles emitted by the radiotracer. There are some preclinical examples of the use of scintillation fibres in animal tissues and blood vessels, but Lucerno Dynamics hopes to build on these studies and develop a new device for future use in humans.
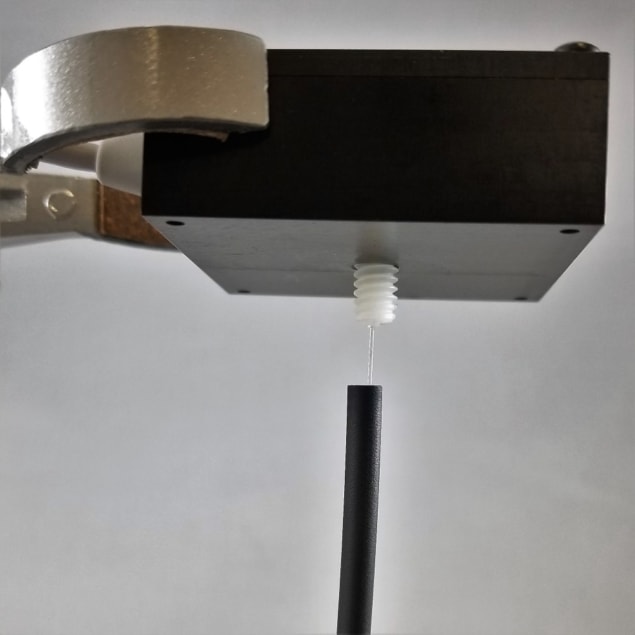
Knowland’s team integrated commercially available scintillation fibres into a venous access catheter. Critical to prototype development was the need to minimize light loss, and so the team developed processes to couple the fibre to a highly sensitive silicon photomultiplier, which converts light into an electrical signal for measurement.
Prototype fibres of two different diameters (0.25 and 0.50 mm) were inserted into artificial veins – thin-walled plastic tubing filled with varied concentrations of radiotracer. In this way, the team tested performance characteristics such as linearity and sensitivity, as well as the inner-vein-signal to outer-vein-“noise” ratio.
“We were able to successfully measure radiotracer concentration within the artificial veins,” says Knowland. “The device output was linear over the range of concentrations expected clinically and it produced approximately 450 counts per second per mega-Becquerel per millilitre.”
A scintillating future
Lucerno Dynamics are highly positive about these initial feasibility tests, but point out a number of challenges they are working to overcome. “It was more difficult than we anticipated to position and align the fibres within the artificial veins. When a fraction of a millimetre can matter, you must be very precise and careful,” Knowland explains. The team also aims to increase device sensitivity and investigate a greater range in vein diameters.
Another major consideration is that the current standard and best location for measuring radiotracer concentration is in arterial blood, not venous. Arterial access is more difficult and painful, which is why the team have focused on venous access here. “We hope to show that modifications can be made to the kinetic analysis models to allow for the use of venous blood instead,” said Knowland.
The scientists are also working to miniaturize the electronics for clinical use, and hope to soon validate the safety and efficacy in animal, and then human studies. “The device would be a sterile, single-use device that reduces the difficulty of measuring the input function and brings the power of kinetic analysis out of research and into the treatment process for all patients,” said Knowland.