Researchers at the University of Nottingham have demonstrated an optimized method to analyse magnetoencephalography (MEG) data that can improve spatial resolution to the order of 3-5 mm. They achieved this with the aid of an optimized algorithm and custom 3D-printed foam headcasts that restricted participant head movement to about 1 mm. First shown in simulation, then validated in participants who performed a finger movement task, this improved accuracy enabled better measurement of spatial organization of human brain electrophysiology, non-invasively (NeuroImage 10.1016/j.neuroimage.2018.06.041).
What is MEG?
MEG measures the magnetic fields generated by neural current flow in the brain with high (millisecond) temporal resolution, allowing measurement of very fast time-frequency dynamics of brain networks. This is unlike brain imaging techniques such as functional MRI (fMRI), which rely on a lagged, indirect blood oxygenation measure of neural activity, making it difficult to capture fast events.
Spatial mapping of electrical sources using MEG is, however, complicated since there are many more voxels in the brain than there are magnetic field sensors in the scanner. This makes the inverse problem (inferring source distributions based on extracranial fields) non-unique, and hence requires the use of complicated localization algorithms, such as beamforming. Blurring of the measured fields due to having fewer sensors than current sources is exacerbated by participant movement. In this work, the researchers minimized blurring due to the inverse problem by optimization of the beamforming algorithm, while head movement was mitigated using the foam headcasts.
In the healthy brain, movement (of a finger, for example) causes measurable changes in brain waves, with a decrease of beta waves (13-30 Hz) during movement, followed by an increase above baseline after movement cessation. The latter effect, the so-called rebound, is thought to be a marker of neural inhibition and is spatially specific.
Simulation studies
In simulations, the researchers modelled two point sources with time courses that were alternately on and off in response to theoretical stimulation. By segmenting the MEG data into two datasets and calculating separate beamformer weights (for spatial localization), rather than beamforming the whole dataset, the spatial separation of the sources was improved significantly.
In other words, removing sources of no interest left fewer sources to be minimized by the beamformer equation, leading to a sharper localized peak. However, the team suggest that there should be sufficient data to allow this segmentation; for a detectable signal, many MEG trials must be acquired.
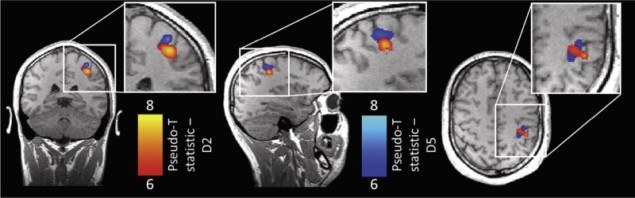
For the experiments, participants performed multiple runs in the MEG scanner while tapping either their index (D2) or little (D5) finger, with rests in between each tap. A similar task was performed using fMRI, for comparison.
Since the same participants underwent fMRI and MEG, the maps of digit representations in the brain from the two neuroimaging modalities could be overlaid on the same anatomical image. The researchers could then calculate distances between MEG D2 or D5 and fMRI D2 or D5 in the same space.
Digit distances
The researchers found that the representations of the index (D2) and little (D5) finger in the cortex of individual subjects could be separated spatially using MEG, even when separation distances were only about 3-5mm. The beta rebound was shown to be mapped topographically in accordance with well characterized topography of the sensorimotor cortex. Furthermore, they showed significant overlap between MEG and fMRI.
However, the overlap between blood oxygenation level dependent (BOLD) fMRI and MEG responses were not perfect – the distances between D2 and D5 in each modality were different, with fMRI giving larger distances. This may be due to assumptions in the MEG models used to derive sources. Alternatively, the differences may be neurophysiological in origin: the BOLD activity may be more spread out, encompassing somatosensory (touch) and motor regions, whereas the beta rebound may be more specific.

The authors note that the construction of custom headcasts is expensive, and that this should be taken into account for large cohort studies – although the benefits to data quality may outweigh the costs.
This research showed that MEG has the required spatial resolution to delineate separate digit responses in the same hand, using the beta rebound; first in simulation and then validated experimentally. MEG therefore has an untapped potential in spatial resolution, which could be exploited in patient cohorts with possible sensorimotor alterations, for example focal hand dystonia (writer’s cramp).



