The Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting, held this week in Philadelphia, PA, brought together physicians, technologists, pharmacists and scientists from around the globe to share research and collaborate on “Imaging the Future of Human Health”. The meeting included 850 scientific presentations and nearly 1000 posters on cutting-edge research advances. Here are just a few of this year’s highlights.
Targeted radionuclide therapy enhances immunotherapy
A research team from the University of Wisconsin Madison demonstrated that combining targeted radionuclide therapy (TRT) with immunotherapy may improve survival of patients with metastatic melanoma. External-beam radiotherapy has been shown to enhance immunotherapy response in preclinical studies, but results can be limited due to the presence of metastatic disease. This study showed, for the first time, that TRT can successfully synergize with immunotherapies.

The researchers injected melanoma-bearing mice with 86Y-labelled NM600 and performed PET/CT scans 3, 21 and 48 hr later. They used these images to determine the activity of the TRT agent 90Y-NM600 required to deliver the desired radiation dose to the tumour. They then treated groups of mice with TRT, followed by anti-CTLA-4 immunotherapy at days 4, 7 and 11.
“Following intravenous injection of our TRT agent, it undergoes selective tumour uptake and prolonged retention, allowing for the precise delivery of radiation dose to tumours wherever they are in the body – something that is unique to this form of radiation treatment,” explains first author Reinier Hernandez. “We have also demonstrated a low toxicity profile for normal organs and tissues at the low immunomodulatory radiation doses of NM600.”
Mice treated with either 90Y-NM600 or anti-CTLA-4 alone showed a dose-dependent decrease in the rate of tumour progression, but not tumour regression. Mice receiving the combination of 90Y-NM600 and anti-CTLA-4 showed tumour regression and improved survival compared with other treatment groups, with 66% exhibiting a durable complete tumour response.
PET reporter gene/probe monitors success of gene therapy
Gene therapy for diseases of the central nervous system is a growing field, but progress is limited by the absence of imaging techniques to monitor delivery or expression of the therapy. A new PET reporter gene/probe system makes it possible, for the first time, to noninvasively monitor the level and location of gene expression in all areas of the brain, providing an early indication of the likelihood of treatment success.

The researchers, from Stanford University, examined the use of pyruvate kinase M2 (PKM2) as a PET reporter gene, and imaged its expression with the radiotracer 18F-DASA-23. Developed in the Gambhir lab, 18F-DASA-23 is a novel reporter probe that can cross the blood-brain barrier and targets PKM2 in the central nervous system.
In the study, the researchers infected mice with an associated-adeno virus (AAV) containing the PKM2 reporter gene. They then imaged the mice with 18F-DASA-23 over two months to observe the increase in PKM2 expression. Results, confirmed by 18F-DASA-23 uptake studies and mRNA analysis, showed a good correlation between PKM2 and the radiotracer. Further analysis showed an increase in PKM2 expression in infected mice when compared with controls.
“Having a reporter gene/reporter probe system that allows monitoring of all areas of the brain opens the door to more accurate and less invasive imaging of the brain and of gene therapies used to tackle diseases of the brain,” says first author Thomas Haywood.
Fluciclovine PET/CT locates recurrent prostate cancer
Adding 18F-fluciclovine PET/CT to the diagnostic work-up of patients with biochemical recurrence of prostate cancer can find previously undetected lesions in the prostate and other tissues. The scan changed treatment management for the majority of patients, according to results of the LOCATE trial, a prospective multicentre study conducted at 15 sites in the US.
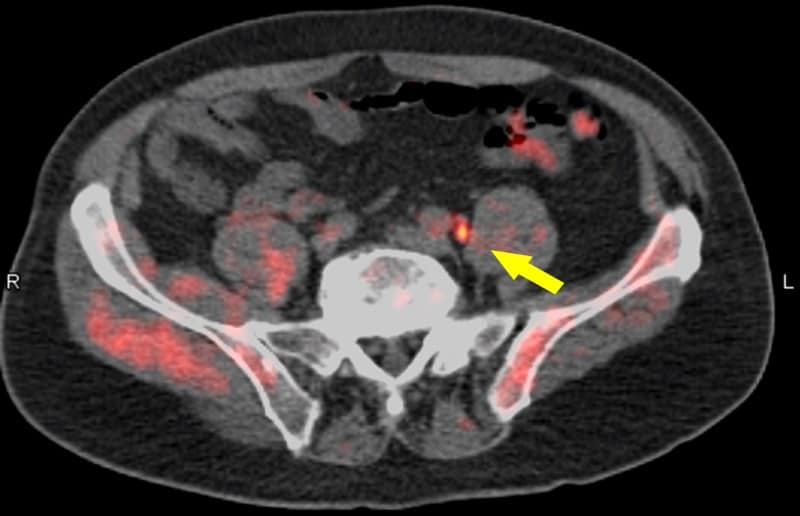
Up to 30% of patients with prostate cancer will develop local or distant recurrence within 10 years of radical prostatectomy or radiotherapy. Determining the location and extent of recurrent disease helps optimize the selection of appropriate management. Current anatomical imaging procedures, however, have limitations in identifying the sites of recurrence.
For the LOCATE trial, 213 men with biochemically recurrent prostate cancer were evaluated with 18F-fluciclovine PET/CT, after having negative or equivocal findings on conventional imaging, such as a bone scan, CT or MRI. Results showed that 59% of patients had their clinical management changed by the 18F-fluciclovine findings, with 78% of these changes classified as “major”, meaning a change in treatment modality.
“Selecting appropriate treatment for men with recurrent prostate cancer is critical,” explains Austin Pantel of the University of Pennsylvania. “Many options are available, and additional information, such as that provided by 18F-fluciclovine PET/CT, may help tailor personalized treatment plans.”
Alpha emitter targets wide range of solid tumours
In 2017, researchers from Memorial Sloan Kettering Cancer Center developed a novel approach to pretargeted radioimmunotherapy (DOTA-PRIT) that demonstrated, preclinically, complete responses in several solid tumour types using the beta-emitting 177Lu-DOTA-hapten. Now, they have expanded this approach to 225Ac, an alpha-emitting isotope.

“Targeted alpha radiotherapy has shown considerable promise for patients, especially for those with advanced castration-resistance prostate cancer,” explain Steven Larson and Sarah Cheal. “By combining DOTA-PRIT with 225Ac-proteus-DOTA hapten, we can potentially target a wide array of cancer types for which we have validated DOTA-PRIT bispecific antibodies.”
DOTA-PRIT has a major advantage over other forms of radioimmunotherapy because of its high ability to deliver radiation to tumours while sparing normal tissues, such as kidney and bone marrow. The researchers synthesized proteus-DOTA, radiolabelled it with 225Ac, and conducted in vitro and in vivo studies of a mouse model with colorectal cancer. They also performed a toxicity study in tumour-free mice with varying doses of 225Ac-proteus-DOTA.
The team found that the new approach, 225Ac-proteus-DOTA, mimics the behaviour of 177Lu-DOTA-hapten, with high tumour uptake, minimal accumulation in normal tissue, good whole-body clearance, no acute toxicity and no chronic radiation damage. It also offers greater versatility for treating a wide variety of solid tumours.



