
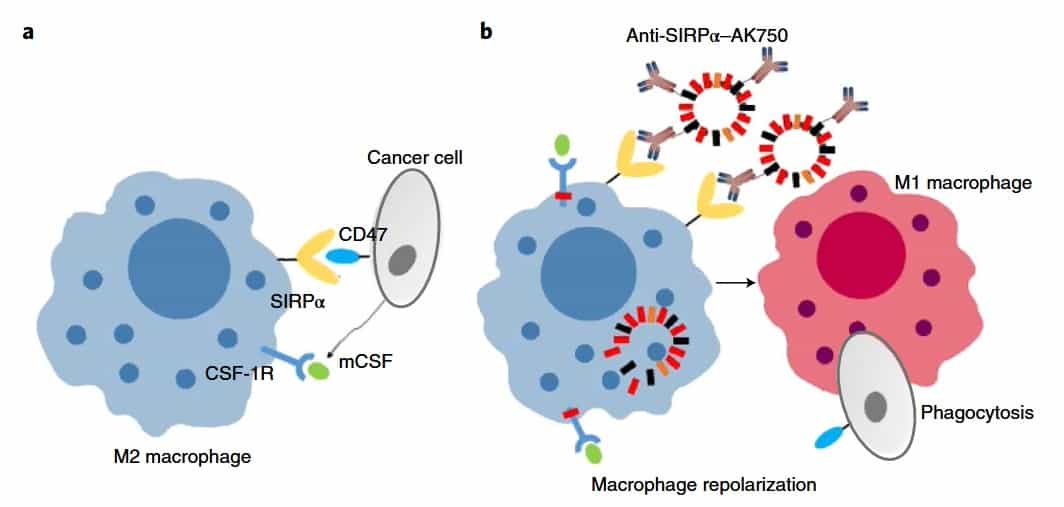
Macrophages are key players in the body’s immune response and they can effectively “eat” cancer cells. However, cancer cells are tricky and express a “do not eat me” signal to avoid this phagocytosis. Moreover, cancer cells can switch the tumour-associated macrophages from an antitumour M1 phenotype to an M2 pro-tumourigenic one. This transition from M1 to M2 is ensured by CSF-1R (a tyrosine kinase receptor found on the surface of macrophages).
Inhibition of CSF-1R signalling is thus starting to be seen as an attractive therapeutic goal. A team of scientists from Brigham and Women’s Hospital have managed to show that a supramolecule assembly termed AK750 enabled a shutdown of the CSF-1R-signalling pathway and enhanced M2-to-M1 repolarization within the tumour microenvironment. The supramolecule improved antitumour and antimetastatic efficacies in animal models of melanoma and breast cancer, paving the way for a new kind of immunotherapy (Nature Biomed. Eng. 2 589).
The birth of the supramolecule AK750
Initially, the authors simulated the formation of the supramolecule in silico. They then synthesized amphiphile subunits (which have both hydrophilic and lipophilic properties) and, in agreement with the theoretical predictions, a stable supramolecular assembly formed.
In the next step, the authors exposed macrophages to AK750. They observed a sustained inhibition of CSF-1R, even after 48 hours. Moreover, a reverse transcription polymerase chain reaction (RT-PCR) analysis revealed increased expression of IL12 and IL10 (pro-inflammatory molecules that play an important role in host defence and immune homeostasis) in the macrophages following AK750 treatment – indicating that these are being polarized to M1 status.
Further on, the treatment of macrophages with IL4, which can polarize a macrophage to an M2 state independent of CSF1 signalling, resulted in a significant increase in the M2 phenotype. When these M2 macrophages were treated with AK750, there was a significant increase in M1 macrophages, indicating the efficient repolarization of M2 macrophages to M1 phenotype. Importantly, M2 status can be achieved by the cancer cell through manipulation of the tumour microenvironment, but AK750 treatment can inhibit these modulatory effects.
The effect of AK750 in vivo
The authors tested the in vivo efficacy of the AK750 supramolecule in melanoma and breast cancer animal models. The treatment of melanoma-bearing mice with AK750 resulted in a complete inhibition of tumour growth, revealing an inhibition of the CSF-1R phosphorylation and a reduction in the M2 macrophages, together with an increase of the M1 pool. For the breast cancer model, AK750 significantly inhibited tumour growth, but in a dose-dependent manner.
The “do not eat me” signal that cancer cells express is called CD47 and binds to a signal-regulatory protein α (SIRPα) receptor on macrophages to prevent phagocytosis. The authors hypothesized that integrating a SIRPα-targeting function could increase the antitumour efficacy. Thus, they integrated tumour-associated macrophages isolated from melanoma-bearing mice with the bifunctional supramolecule. An internalization of the supramolecule was identified within the macrophages, together with a significant inhibition of CSF-1R phosphorylation and an increase of the M1 phenotype.
The findings of this study indicate that bifunctional supramolecules could be used to advance immunotherapy in humans. The supramolecule can accumulate in the tumour and induce a sustained inhibition of the CSF-1R signalling cascade, resulting in enhanced antitumour efficacy and reduced metastasis in aggressive tumour models. Moreover, blocking two distinct targets in the same immune cell might be the future of immuno-oncology.



