Researchers in Korea and the US have engineered a light-activated synthetic synapse that enables an artificial muscle to move and contract. The newly designed organic optoelectronic synapse converts light to electrical signals to enable an artificial muscle to flex and stretch (Science Advances 10.1126/sciadv.aat7387).
Mimicking human sensory and motor functions has become one of the key motivations for bio-inspired engineering, especially for developing electronic prostheses and neurorobotics. Performing human-like sensing functions, as well as processing neural signals including motor responses, are among the key tasks for an artificial sensorimotor nervous system. However, the major focus to date has been on the development of materials and tools that mimic memory properties in a human brain at the basic level. Therefore, emulation of the human neuromuscular system remains a challenge.
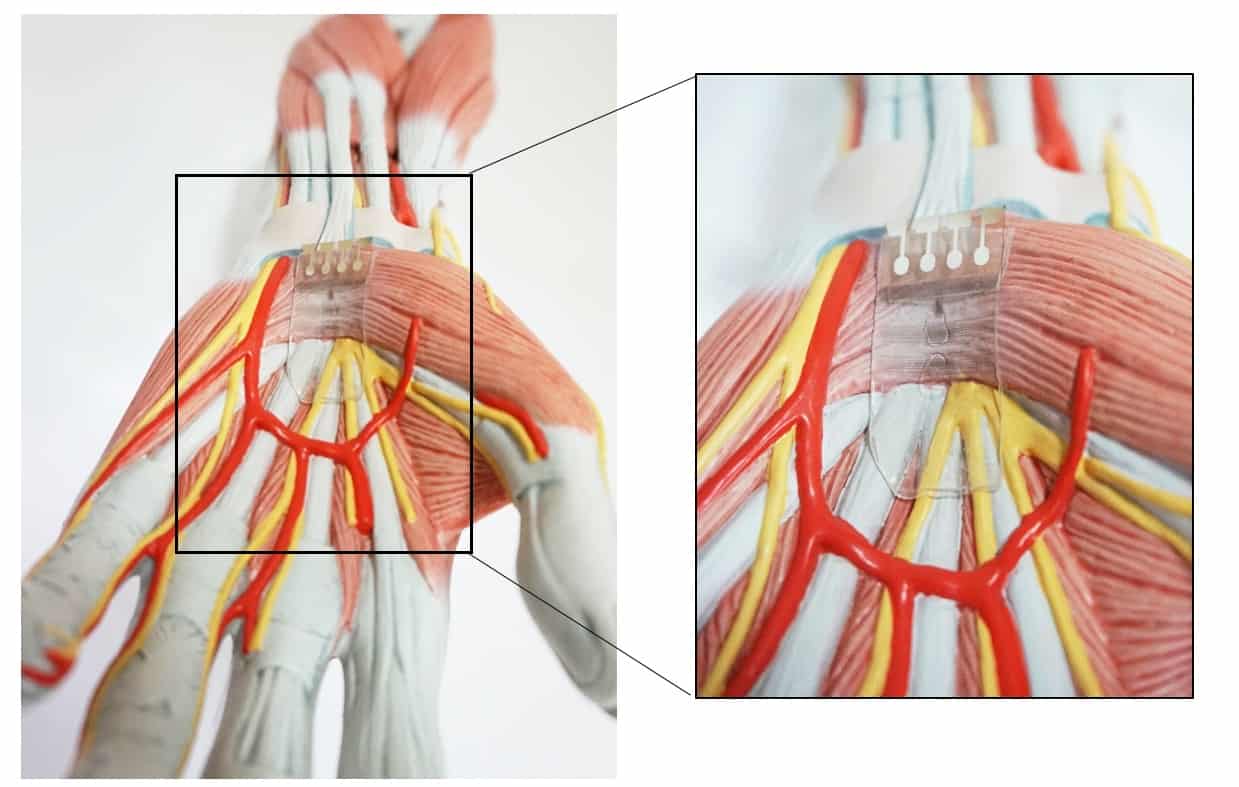
Stretchable optoelectronic neuromuscular system
Taking the neurorobotics field a step further, the researchers — at Seoul National University and Stanford University, led by Tae-Woo Lee and Zhenan Bao — designed a synthetic optoelectronic synapse. This sensorimotor system contains a photodetector and organic synapses that contract an artificial muscle when triggered by pulses of light. Their design is similar to optogenetic techniques used in biology, where genetically modified cells and neurons become sensitive to light to operate.
The new design is a stretchable organic nanowire synaptic transistor (s-ONWST) that propagates optical signals and converts them into excitatory postsynaptic currents (EPSCs) that activate contraction of an artificial muscle. To achieve this design, the researchers aimed to develop a device that will be flexible, stretchable and durable under different movement conditions. Hence, they chose organic nanowires, which can achieve the flexibility of a biological neuromuscular system more easily than conventional rigid inorganic artificial synapses.
The team fabricated the polymeric organic nanowire-based artificial synapse using ion gel electrolyte and a single organic nanowire. Next, they added a photodetector to the s-ONWST and assembled the optoelectronic synapse. Upon exposure to light, the photodetector generates voltage spikes and drives the s-ONWST to emit the EPSCs.
The transistor’s ion gel-based electrochemical behaviour was confirmed through current–voltage curves. In biological synapses, neurotransmitters migrate across the synaptic cleft (the small space between the presynaptic neuron and the postsynaptic neuron) upon receiving an action potential. In a similar manner in the artificial neuromuscular system, negative presynaptic voltage spikes move anions towards the organic nanowire.
The researchers determined that their organic optoelectronic synapse has the potential to be used as an optical wireless communication method in human–machine interfaces. Their experiments showed that the s-ONWST can react to visible light displayed in patterns similar to Morse code. In other words, every single English letter elicited a distinguishable EPSC amplitude response. Other tests on the s-ONWST further confirmed that stretching the device did not result in any significant defect in its response.
Bio-inspired soft electronics can take advantage of light-interactive actuators to drive operation of sensory and motor systems. This would be achieved through the development of optical wireless communication in which undirected visible, infrared or ultraviolet light propagates a signal.
“We have demonstrated the first neurologically inspired organic optoelectronic sensorimotor synapse,” the authors claim. They are looking forward to the emergence of “a promising strategy for the development of next-generation biomimetic soft electronics, soft robotics, neurorobotics and electronic prostheses” in the near future.