Tissue engineering is an emerging field in which cells, biomaterials and biotechnologies are employed to replace or regenerate damaged or diseased tissues. Currently, this is achieved by generating a biomaterial scaffold outside of the body, maturation in a bioreactor and then surgically implanting the created tissue into the patient. This surgery, however, poses the added risk of infection, increases recovery time and may even negate the therapeutic benefits of the implant.
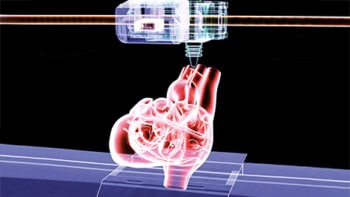
To prevent such complications, a US research team is developing a way to fabricate 3D tissue scaffolds inside a living patient – so-called intracorporeal tissue engineering. The researchers, from the Terasaki Institute, Ohio State University and Pennsylvania State University, aim to use robotic direct-write 3D printing to dispense cell-laden biomaterials (bioinks) in a highly precise, programmable manner. The printed bioinks are delivered through minimally invasive surgical incisions and the body itself acts as the bioreactor for maturation.
Any technique used to directly print tissues inside the body, however, must meet a specific set of requirements. The biomaterial must be 3D printable at body temperature (37 °C), for example, and all procedural steps should not harm the patient. For example, current methods use UV light to crosslink the constructed tissue, which is not safe for use within the body.
To meet these requirements, the team produced a specially-formulated bioink designed for printing directly in the body. They used the hydrogel gelatin methacryloyl (GelMA) as the biomaterial, and introduced Laponite and methylcellulose as rheological modifiers to enhance printability. “This bio-ink formulation is 3D printable at physiological temperature, and can be crosslinked safely using visible light inside the body,” explains first author Ali Asghari Adib.
The researchers used the GelMA/Laponite/methylcellulose (GLM) formulation, with and without encapsulated fibroblasts, to construct complex 3D tissue scaffolds with clinically relevant dimensions and consistent structures. They successfully 3D printed the scaffolds on agarose and chicken breast pieces, using on-site crosslinking with visible light. For cell-laden GLM, the fibroblasts exhibited consistent mechanical properties and a viability of 71–77% over 21 days in the printed scaffolds.
Another challenge of intracorporeal tissue engineering is attaching the printed structure onto soft, live tissue surfaces. For this, the researchers employed a unique interlock technique using the robotic 3D printer. They modified the nozzle tip to penetrate 1.6 mm into the soft surfaces and fill the punctured space with bioink as it withdrew, thus creating an anchor for the tissue construct. In experiments on agarose and chicken pieces, this interlocking mechanism created stronger attachment of the scaffolds to the tissue. The team observed 3.5-fold (chicken) and 4-fold (agarose) increases in the biomaterial–tissue adhesion strength compared with printing onto the tissue surface.

Print me an organ
The researchers conclude that the GLM biomaterial and robotic interlocking mechanism pave the way towards intracorporeal tissue engineering. This could provide lower-risk, minimally invasive laparoscopic options for procedures such as 3D printing of bio-functional hernia repair meshes, implanting patches to enhance ovarian function, creation of cell-laden scaffolds to repair tissue or organ defects, and delivery of drug-loaded or growth factor-tethered biomaterials to improve tissue regeneration.
“Developing personalized tissues that can address various injuries and ailments is very important for the future of medicine,” says Ali Khademhosseini, director and CEO of the Terasaki Institute. “The work presented here addresses an important challenge in making these tissues, as it enables us to deliver the right cells and materials directly to the defect in the operating room.”
The researchers report their findings in Biofabrication.