Researchers in the US have developed a novel optical microscopy technique that offers new insights into amyloid plaques, which are characteristic of neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. Better understanding of these clumped or misfolded proteins could help develop new therapies, the scientists claim.
Amyloids are insoluble, abnormal protein aggregates that have been linked to the development of various diseases, including diabetes and neurological conditions such as Alzheimer’s, Parkinson’s and Huntington’s disease. While most amyloid proteins may be non-toxic, they become problematic when they form fibrous deposits, or plaques, around cells and disrupt their normal function. In the brain, the misfolding and clumping of amyloids can kill many neurons.
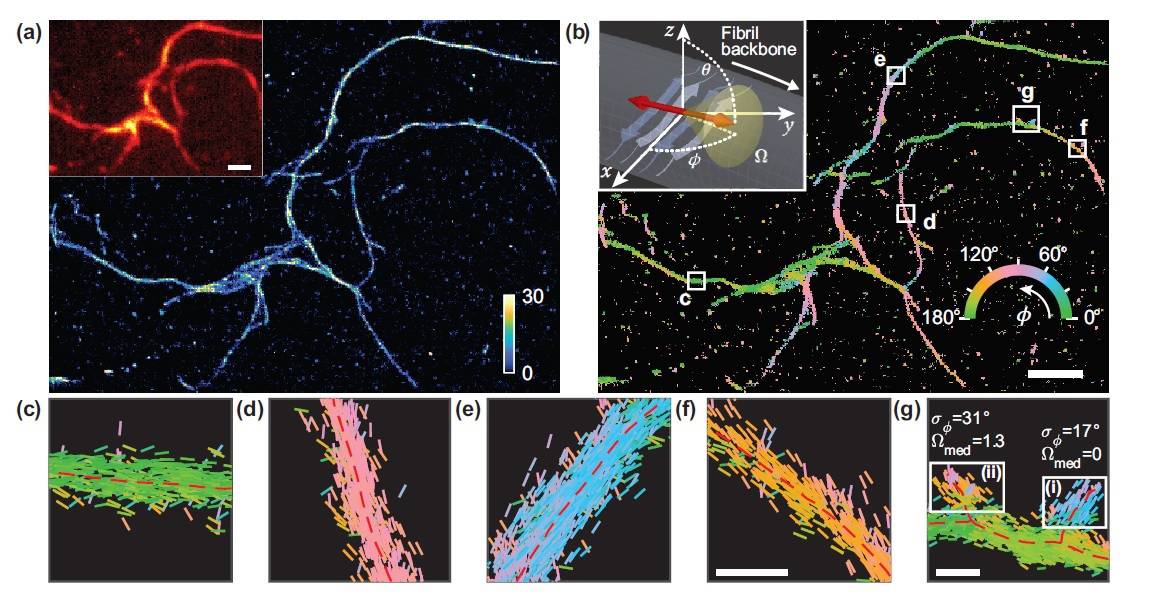
Understanding the underlying structure of these plaques could pave the way for the development of effective therapeutics against these diseases. Now, researchers at Washington University in St. Louis have developed a new optical microscopy technique that measures both the location and orientation of single molecules in these amyloid protein aggregates, revealing nanoscale details about their structures.
“We need imaging technologies that can watch these molecular movements in living systems to understand the fundamental biological mechanisms of disease,” explains Matthew Lew, who led the research. “Amyloid and prion-type diseases like Alzheimer’s, Parkinson’s and diabetes are our first targets for this technology, but we see it being applied in many other areas too.”
Amyloid proteins have the ability to be stained by certain florescent dyes. And, as there is no artificial link between the fluorescent probes and amyloid surfaces, the probes’ binding orientation can potentially provide information about the structure and organization of the amyloid proteins.
The researchers created a performance metric to characterize how well various microscopy techniques measured the orientations of such fluorescent dyes. In work described in the journal Optica, they report that a microscope that splits fluorescence light into two polarization channels provides superior and practical orientation measurements.
The new super-resolution microscopy method allows them to measure not only the location of the fluorescence, but also characteristics such as polarization, which are ignored in most other microscopy approaches.
“The metric we developed calculates the performance of a particular microscope design 1000 times faster than before,” explains Tingting Wu. “By measuring the orientations of single molecules bound to amyloid aggregates, the selected microscope enabled us to map differences in amyloid structure organization that cannot be detected by standard localization microscopes.”
The researchers quantified how the orientations of fluorescent molecules (Nile red) varied each time one attached to an amyloid protein. Differences in these binding behaviours can be attributed to structure differences between amyloid aggregates. Because the method provides single-molecule information, the researchers could observe nanoscale differences between amyloid structures.
Imaging techniques shed light on amyloid fibrils associated with Alzheimer’s disease
“In optical microscopy and imaging, scientists and engineers have been pushing the boundaries of imaging to be faster, probe deeper and have higher resolution,” Lew says. “Our work shows that one can shed light on fundamental processes in biology by, instead, focusing on molecular orientation, which can reveal details about the inner workings of biology that cannot be visualized by traditional microscopy.”
The researchers note that their microscopy setup used commercially available parts that are accessible to anyone performing single-molecule super-resolution microscopy. Next, they plan to monitor amyloid structures over hours and days to observe nanoscale changes as they develop and organize. Long-term studies of amyloid aggregates could reveal new information about how amyloid proteins are organized and how quickly they grow or spontaneously dissolve, the team says.



