Researchers at Kyoto University, the National Institutes for Quantum and Radiological Science and Technology (QST) and the University of California Irvine observed that tumour masses containing iodine-infused nanoparticles shrank when they were hit with single-energy X-rays. Their in vitro laboratory studies are described in Scientific Reports.
Cancer cells and the photoelectric effect
“Cancer radiation therapy is rapidly changing. Utilization of high-Z [high atomic number] elements [such as iodine] that can release electrons upon X-ray irradiation can bring further advances in radiation therapy,” says senior author Fuyuhiko Tamanoi from Kyoto University.
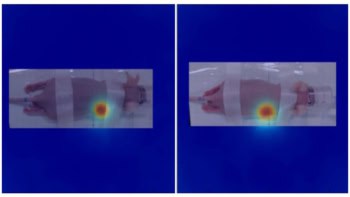
Tamanoi’s team designed and produced silica-based nanoparticles containing iodine and introduced the nanoparticles to one-millimetre in vitro masses of human ovarian cancer cells, human head-and-neck cancer cells and human brain cancer cells. The nanoparticles accumulated in the cancer cells. Tumour masses containing the nanoparticles shrank when bombarded by 33.2 keV X-rays for 30 minutes.
The researchers believe that the reduction in tumour size can be traced back to the photoelectric effect, in which electrons including Auger electrons are released when X-rays hit iodine atoms. They also hypothesize that these Auger electrons interact with the DNA in the nuclei of cancer cells, resulting in double-strand breaks in the DNA that trigger apoptosis, or programmed cell death.
The researchers studied how the nanoparticles accumulate in cancer cells by tagging some nanoparticles with red fluorescent markers and observing their uptake with confocal microscopy. They also confirmed that cells were dying by programmed cell death rather than other mechanisms using a laboratory test that detects DNA damage at late stages of apoptosis.
The future of radiation therapy?
While the iodine-infused nanoparticles can also be taken up by healthy cells, the researchers believe that their accumulation in tumours in animal models confirms preferential uptake in cancer cells. They hypothesized that this happens because tumours are highly vascularized and have porous blood vessel walls, so the nanoparticles leak out of blood vessels and could accumulate in tumours.
The research team also observed that irradiating the tumour masses with 33.2 keV X-rays shrank the masses the most.
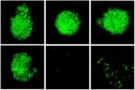
Gd-loaded nanoparticles plus monochromatic X-rays can destroy tumours
“While a sharp peak at 33.2 keV [compared with other energies] can be explained by the absorption of energy by K-shell electrons, it was surprising to observe the relatively sharp decrease in tumour destruction effect beyond 33.2 keV,” says Tamanoi. “We speculate that this was due to multiple electron release events taking place after X-ray irradiation.”
The researchers are continuing their investigations to learn more about the photoelectric effect, how their nanoparticles behave inside cancer cells and in simulation studies, and the implications of both for the development of Auger therapy.