A new imaging technique has allowed researchers in the UK to create a 3D map that charts the flow of blood through a living zebrafish. Andrew Harvey and colleagues at the University of Glasgow used an optical setup that produces pairs of “Airy beams” corresponding to individual microscopic beads flowing in a fish’s blood. Their approach could lead to new and better ways to explore the characteristics of microscopic biological systems.
The spatial resolution of a conventional optical microscope is about 300 nm, which is on par with half wavelength of visible light. While smaller structures can be observed, their spatial features are blurred. In simple 2D systems, the blurring can be compensated for by locating the centres of blurred objects and constructing “point spread functions” (PSFs) on top of them. This can reduce blurred objects to single points of light to within about 10 nm precision – which is important when tracking single, fluorescently-labelled molecules in biological systems.
PSFs can also be used for simple 3D systems, since their shapes can indicate their depths, or axial distances relative to the imaging apparatus. However, the technique becomes far less effective when imaging 3D groups of objects, which often results in overlapping PSFs that are far more difficult to analyse.
Curved light
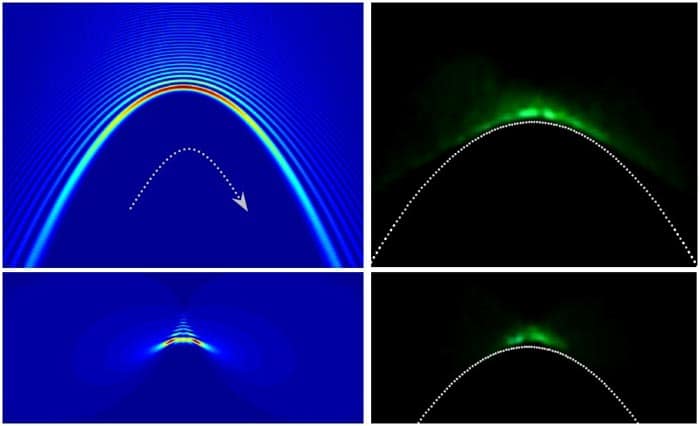
Harvey and colleagues have been developing a better 3D method that uses imaging optics to transform a PSFs into an “Airy beam”, a waveform that does not spread out over time and appears to curve as it travels. The shape of the Airy beam depends on the axial distance to the object, allowing the depth of the object to be determined. While this technique is effective, it can be difficult to implement.
In their latest research, the team introduce an even more effective optical setup that is easier to calibrate. Their system converts a PSF into a twin Airy beam that appears as two spots on either side of the object. The separation between the spots increases with axial distance increases and therefore measuring the separation gives the depth of the object. This approach enabled the researchers to locate fluorescent nanocrystal beads to within 30 nm, across an axial range of over 7 micron.
Light bends itself round corners
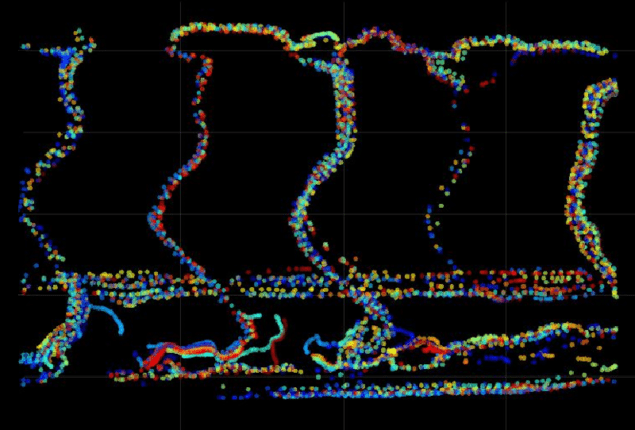
As a proof of concept, they used the technique to observe the motions of 1 micron fluorescent beads when injected into the blood of living zebrafish –tracking their twin Airy beam PSFs at 26 frames-per-second. With high enough bead densities, Harvey and colleagues could clearly map out the 3D shapes of the fish arteries over a depth range of 0.1 mm.
The technique could soon offer significant new opportunities for optically imaging microscopic structures, including living systems on a cellular level. Harvey’s team describes how nanobeads could be made to fluoresce in the presence of oxygen or acidic conditions; or loaded into soft gels that are deformed by growing cells, potentially yielding new insights into the mechanical forces they exert.
The imaging technique is described in Physical Review Letters.