Improved radiotherapy techniques and technologies allow an increasing number of children diagnosed with cancer to survive long into adulthood. Unfortunately, the very treatments that save their lives are associated with several severe, life-threatening, or even fatal chronic health conditions. For example, children who received radiation therapy are at a heightened risk of developing heart disease later in life. Further improving radiation treatments to minimize this risk requires a deep understanding of the relationship between radiation exposure and heart disease that is currently lacking. A multi-institutional team of researchers in the US has enhanced a critical computational tool to enable new insights into this relationship.
Our current understanding of how radiation exposure contributes to heart disease comes from several studies of childhood cancer survivors. These studies, which correlated radiation dose to the heart with various cardiac conditions, demonstrated a positive correlation, or that risk generally increases with increasing cardiac dose. All these studies, however, considered the whole heart as a single unit.
In reality, the heart is a complex organ made up of several substructures including valves, arteries, ventricles and atria. Similarly, heart disease refers to a diverse collection of conditions, including narrowed or blocked blood vessels and heart rhythm problems, among others. Recent evidence suggests that each of these unique conditions is associated with radiation dose to specific heart substructures, rather than to the heart overall. Existing research infrastructure, however, is insufficient to elucidate these associations.

Thus, Rebecca Howell led a team of researchers from six institutions to enhance that infrastructure. First author Suman Shrestha, doctoral research fellow in Howell’s Late Effects Research Group at The University of Texas MD Anderson Cancer Center, was awarded a career development award from the Childhood Cancer Survivor Study (CCSS) to tackle this problem. The researchers published their findings in Radiotherapy & Oncology.
Organ doses for historic radiotherapy
Correlating radiation therapy exposures to observed health effects requires comprehensive knowledge of the delivered treatment. Critically, studies of long-term health effects rely on historical medical records that often lack computed tomography (CT) scans of the patients. Therefore, researchers must simulate the treatments on representative patient surrogates, known as phantoms, to estimate the delivered dose.
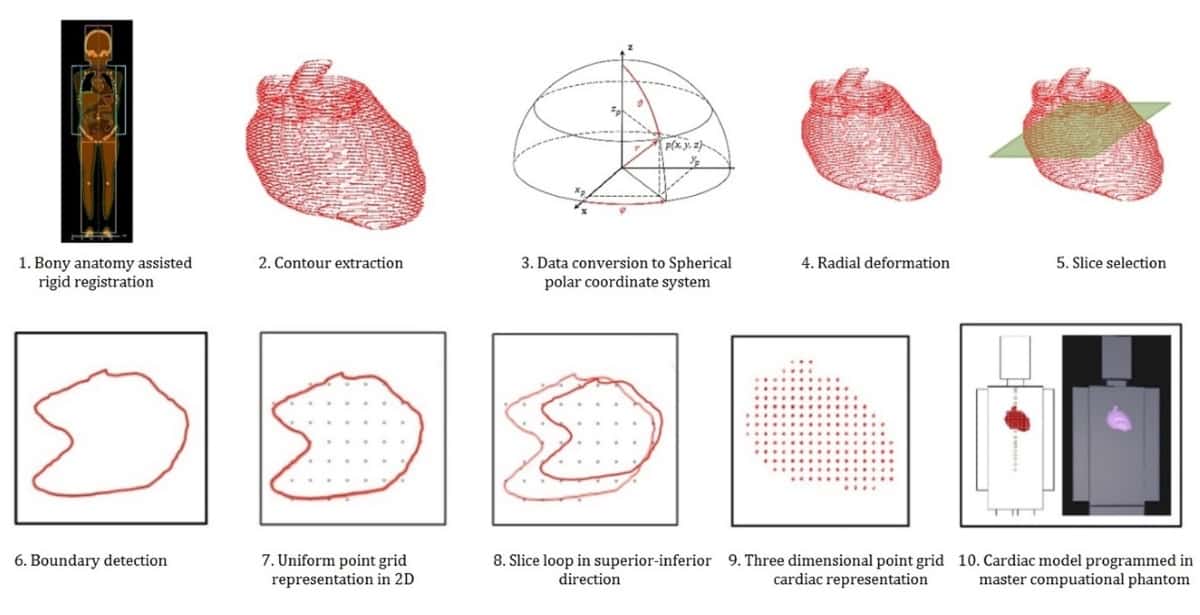
Howell’s lab at MD Anderson performs these simulations using a computational phantom that can be scaled in size based on the patient’s age at the time of treatment. This scaling is particularly important for simulating paediatric cancer treatments because of the large variation in size from infancy to early adulthood. The heart model in this age-scalable phantom, however, represented the heart as a single structure and did not delineate its distinct substructures.
The University of Florida (UF) and National Cancer Institute (NCI) maintain a different set of computational phantoms that include 10 heart substructures, representing the most comprehensive heart model available for radiation simulations. This set of phantoms, however, only includes six distinct ages and cannot be scaled to match a particular patient’s age at treatment.
Their powers combined
The research collaboration combined the age scalability of the MD Anderson phantom with the detailed completeness of the UF/NCI phantoms to create a hybrid heart model. Their new heart model includes the 10 heart substructures represented in the UF/NCI phantoms, as well as four additional heart substructures, for a total of 14 substructures that can be scaled to any age.
The team tested the hybrid heart model in three ways and found it to be anatomically accurate across children ranging from infants to adolescents, clinically acceptable and dosimetrically viable – all crucial factors for widespread research use.
Moreover, the methods used to enhance this heart model can be applied to other organs and tissues to further improve outcomes research. “This work was paradigm shifting for our team in how we will approach organ development and validation in future studies,” says Howell. “We are using methodologies similar to those developed here to develop additional organs in our computational phantom, for example, the colon, with the ascending, transverse and descending colon substructures defined.”
Caution required when balancing lung versus heart radiotherapy dose
The new heart model is already enabling researchers to consider the unique relationships between radiation exposures and complications associated with specific heart substructures.
“We have used the heart model developed in this study to calculate dose to [the] heart and its substructures for over 13,000 survivors in the CCSS treated with radiotherapy. Analyses are ongoing to determine relationships between specific late cardiac diseases and dose to specific substructures, for example, relationships between coronary artery disease and coronary artery dose,” says Howell.
This understanding will facilitate future studies seeking to refine radiation treatments to avoid distinct heart complications and improve the long-term quality-of-life of cancer survivors.



