Fireworks have become a hallmark of celebrations around the world. Pierre Thebault looks beyond the bright colours and loud bangs, to the array of scientific methods that pyrotechnicians use to improve the safety, environmental impact and spectacle of fireworks
When the clocks strike midnight on New Year’s Eve, the night sky will be lit up with swirling, whistling, flashing, banging, glittering explosions of colour, noise and smoke. Some of these fireworks will be set off in people’s back gardens, but if you really want a good “ooo” and “ahh”, it’s best to head for a professional display. These events, which can cost big money, are a spectacular mainstay of everything from national celebrations such as Guy Fawkes Night in the UK to sporting events like the World Cup and the Olympic Games.
But given that fireworks are “one-shot” products, which function only once, how do professional pyrotechnicians produce such immaculately arranged displays without practice? How do they know what the display will look like if they can’t rehearse it in advance? The answer lies in having a detailed understanding of the composition and behaviour of fireworks. Fortunately, we have learned a lot about these objects, as they are one of the oldest technical activities with which humans have been involved.
Their history dates back to the 14th-century BC, when people in China would throw dried bamboo sticks onto fires to obtain a crackling effect that, they believed, would scare away evil spirits. Such “firecrackers” were, however, not fireworks like those we know and love today. Indeed, it wasn’t until the Tang Dynasty in the 9th century AD that “black powder” – the foundation of fireworks – was developed from charcoal, sulphur and saltpetre. The performance of these mixtures was gradually optimized to 75% saltpetre, 10% sulphur and 15% charcoal – proportions that are still used today, more than 1000 years later.
Over the centuries and around the world, various types of fireworks were invented by talented craftsmen using trial and error, in what was then more “art” than “science”. Flames, sparks, bangs, crackles, and bright exhausts of gas and particles were the main firework effects, but the colours were limited to faded reds and yellows. Of the pyrotechnic compositions – the chemicals in fireworks – 99% were black powder with additives such as iron and ground charcoal mixed in to generate desired visual effects.
A truly scientific approach to fireworks, however, had to wait until the end of the 18th century and the beginning of modern chemistry. In the 1770s French chemist Antoine Lavoisier demonstrated experimentally that combustion was a process in which a substance combines with oxygen. For fireworks, the oxygen was provided by saltpetre, which was identified as potassium nitrate (KNO3) a few years later. Then in 1787 Claude Louis Berthollet – another French chemist – discovered chlorates, which meant that saltpetre was no longer the only oxidizing agent that could be used in pyrotechnic compositions.
Saltpetre’s capacity to provide chemical reactions with oxygen was reported for the first time by French pyrotechnician Claude-Fortuné Ruggieri in the second edition of Elémens de Pyrotechnie, published in 1811. This was a landmark in the science of fireworks, being the first ever treatise explaining pyrotechnic matters in scientific terms and in full compliance with Lavoisier’s theory of combustion. Ruggieri went on to be the first pyrotechnician to use various chlorates and nitrates to give fireworks “true” colours beyond the weak red and yellow. Barium, strontium and copper salts were quickly shown to give green, red and blue respectively.

What’s in a firework?
Enough of the history. What we can now say for sure is that firework effects are produced, projected and propelled in aerial patterns by the chemical reaction of explosive substances that are designed, on ignition, to produce heat, light, sound, gas or smoke.
Most modern-day fireworks contain at their heart a combustible heterogeneous mixture of finely powdered chemicals, which can be tailored to produce a desired display. These pyrotechnic compositions include oxidizing agents (such as chlorates, perchlorates, nitrates and oxides), reducing agents (such as metal powders, charcoal, sulphur, boron and organic compounds), and solid, liquid or paste additives. These additives help to control how fast or slowly a firework burns and make it less sensitive to possible accidental stimuli, such as impact, friction and electrostatic discharge.

Usually the mixture is then pressed, cast, moulded, extruded or rolled to form small spheres, cylinders or cubes intended to burn in the air and move as brilliant coloured points under the dark sky. These objects, known as “stars”, can also include an extra pyrotechnic chemical that breaks them into fragments on burning and modifies the resulting visual effect (figure 1).

The stars are stored in “aerial shells” (figure 2), alongside two other powders. One is a “lift charge” that projects the shells into the air. The other is a “burst charge” that breaks the shell case, ignites the stars and propels them outward. Lying between these two is a column of compressed chemicals that delays the transmission of fire from the lift charge to the burst charge so that the shell explodes at the intended point on its trajectory. Ignited by the lift charge and burning like a cigarette, this delay fuse sets fire to the burst charge after a time interval determined by its burning rate.
All fireworks also need an initial ignition to start the whole process. This is done via a string of textile yarns covered with black powder that is designed to burn progressively along its length with an external flame. This fuse, known as a “black match”, is generally also wrapped loosely in a paper pipe or sheath, which increases its linear burning rate up to several metres per second.
Journey of a firework
Any object that involves fire and explosives comes with a long list of hazards and risks – even something as simple as a child’s sparkler can be dangerous. Fortunately, many restrictions and international standards exist to ensure that those who make, watch and operate fireworks stay as safe as possible. These regulations include construction and performance requirements for consumer and professional fireworks, test methods to verify compliance with such requirements, and labels to help users light fireworks safely and from the right distance. But these regulations have done more than just improve the safety of fireworks – they have also advanced the accuracy of test equipment, which in turn has allowed pyrotechnists to elevate their art. Such equipment is used to chemically characterize the ingredients of pyrotechnic compositions and check their performance before and after they are integrated into fireworks (see box, below).
Testing, testing
There are many performance-controlling parameters in fireworks that need to be regulated and tested. Here are some, why they’re important and the techniques commonly used to test them.
- Particle size and surface area of reactive chemicals determine the burning rate, and are tested using laser diffraction analysis and BET (Brunauer, Emmett and Teller) nitrogen adsorption techniques.
- The surface oxidation state of metal particles also determines the burning rate and is probed using optical and scanning electron microscopy.
- Chemical reactivity is tested using differential scanning calorimetry and thermogravimetry. This property controls burning rate and heat flow.
- Chemical stability and compatibility are important from a safety standpoint, and concern aspects such as storage and the effects of ageing. They are tested by thermogravimetry under vacuum, coupled with gas chromatography.
- Colour quality is analysed using colorimetry and visible/infrared imagery to ensure bright displays.
- Toxicity of reaction products and their decomposition processes are important for minimizing environmental impact and are characterized by gas chromatography and infrared spectroscopy.
One advantage of the chemical testing of fireworks is that it has allowed new compositions to be developed that cut the risks to workers in factories and to users on firing sites. A lot of effort has also gone into ensuring that ingredients and reaction products have as small an impact on the environment as possible. Some companies and laboratories have carried out feasibility and development studies to eliminate heavy metals and other toxic substances, and reduce smoke production and fallouts of unburnt star components – in the EU, these are governed by the REACH environmental regulations. Pyrotechnicians are particularly interested in using organic chemistry to discover substances and molecules that could be safer and more environmentally friendly than the inorganic compounds that fireworks have traditionally used.
But it’s not just their chemical composition that needs to be strictly controlled. How fireworks behave needs to be fully understood too. Take the height a firework reaches – knowing how far it’ll travel is one of the main parameters for determining where users and spectators should be to stay safe. Measuring such “effect” heights commonly involves using theodolites and analysing images recorded by visible or infrared cameras. The possible benefits of using radar and Doppler tracking are also being evaluated to obtain more accurate values of the effect height.
Performing without rehearsals
To help assess the risks the public might be exposed to during firework displays, mathematicians have developed models to predict the ballistic behaviour of aerial shells. These take into account factors such as the deviation angle of mortars – the tubes used to fire shells – from the vertical, and the local weather forecast, especially wind speed. Internal ballistics software – which specifically looks at the propulsion of a projectile – uses real gas equations of state to allow pyrotechnicians to predict the velocity of shells at the muzzle of mortars. Meanwhile, external ballistics software, such as ShellCalc, allows shell trajectories to be plotted in 3D (figure 3).
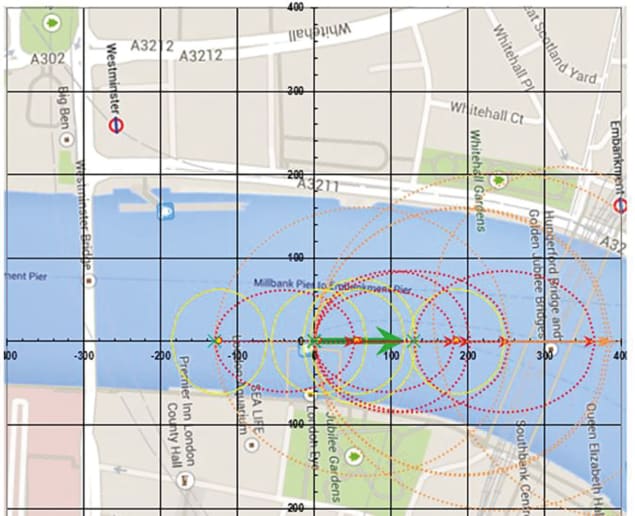
Such modelling programs are set to become a major tool in designing firework displays, especially in places where people, property and historic monuments are relatively close to the firing site. Not only will they suggest optimal orientations of the firework effects, but they will also make it possible to foresee how fallout zones are affected if the weather conditions change. Given that fireworks can be used only once, no-one wants to waste any on a rehearsal. Therefore other software has been developed to let show designers “play” their firework display virtually and make changes to improve the aesthetic quality of the shows, for example to match the firework effects more precisely with music. Being able to plan and visualize a display is a huge advantage for pyrotechnicians, who can create increasingly intricate and ever-more ambitious shows.
With such big displays, it is particularly important to have reliable firing equipment and networks of electric firing lines. They must be resistant to the electromagnetic environment and to bad weather, while being compliant with the latest international electronic standards. They also need to be fired in a precise sequence, from different distances and in time with music. These challenges have led to three solutions: computer-controlled firing equipment; networks of firing lines organized in sub-groups and equipped with electronic hubs linked to a central firing unit by sophisticated lines or radio transmission; and simulation software that can start successive firing sequences during the show.

The science of fireworks
This means that it is now possible to automatically fire complete sequences. However, electronic times are much more accurate than pyrotechnic times and humans must remain in the loop to correct the shifts between the firing program and the aesthetic objectives of the show.
Scientific analysis and understanding of fireworks have led to the truly spectacular professional displays we now know and love. And even if you are simply letting off some fireworks at the end of your garden this New Year’s Eve, it’s nice to know that pyrotechnicians have worked hard to make sure they are safe for you and the environment, as well as being bright and colourful against the night sky.




