Personalized pills that release timed doses of medication tailored to an individual patient’s requirements could improve treatment effectiveness and increase patient compliance. With this goal, researchers at the University of Nottingham have used 3D printing of novel soluble inks to fabricate tablets that deliver drugs with a bespoke dose and release profile. The new technique, described in Materials Today Advances, paves the way for scalable batch production of customizable pills.
Additive manufacturing – or 3D printing – provides a means to fabricate structures with controlled drug release profiles that can’t be created using conventional manufacturing methods. In particular, multi-material inkjet 3D printing (MM-IJ3DP) offers promise for precise deposition of multiple components at production scales. When creating personalized pills, however, the restricted choice of processable materials means that most studies have relied on swelling and diffusion mechanisms for drug delivery, limiting the ability to perform timed release of active material.
Instead, Yinfeng He from the university’s Centre for Additive Manufacturing and colleagues are investigating an inkjet-printable material with a dissolution-based release mechanism. They developed a biocompatible water-soluble ink using acrylomorpholine (ACMO), the photo-polymerized form of which provides a model excipient to enable drug release, and co-printed poly-ACMO with another polymer to create personalized pharmaceutical tablets.
“This breakthrough not only highlights the potential of 3D printing in revolutionizing drug delivery, but also opens up new avenues for the development of next-generation personalized medicines,” says He in a press statement.
Drug delivery
To demonstrate the ability of MM-IJ3DP to create complex composites, the researchers 3D printed a series of 8 mm-diameter structures containing poly-ACMO and another functional polymer. In a single manufacturing step, they fabricated six designs: a disc; an annulus; an insoluble annulus with a soluble core; a soluble annulus with an insoluble core; insoluble cubes within a soluble matrix; and soluble cubes in an insoluble matrix. For each tablet, the soluble component dissolved in phosphate-buffered saline, leaving the insoluble component behind.
The team also fabricated various shaped poly-ACMO tablets with different surface areas, including discs, squares, hexagons, pentagons, pentagrams and four-pointed stars. As expected, the dissolution rate increased linearly with increasing surface area. This trait should enable the team to design structures in which the exposed surface area of the soluble material changes over time.
The next step was to incorporate an active pharmaceutical into the tablets. He and colleagues created poly-ACMO and aspirin-loaded (30 wt%) poly-ACMO inks and co-printed them into a 5 mm-diameter pill with a patterned aspirin-loaded region in the centre. In each layer, the inks were deposited sequentially and cured with UV light in between to prevent cross-contamination.
Raman spectroscopy confirmed successful incorporation of the aspirin in the pill, while NMR spectroscopy of the dissolved tablet revealed a total aspirin loading of 6.9 wt% and a drug loading deviation within typical pharmaceutical quality standards. Further tests showed that the aspirin retained an amorphous state in the printed pill and did not recrystallize after ink curing.
Timed doses
Finally, He and colleagues designed 8 mm-diameter tablets with different patterns of insoluble and soluble ink to create a range of release rates. They 3D printed four designs: an aspirin-loaded poly-ACMO tablet with a small insoluble core; a tablet capped top and bottom with insoluble surfaces; a capped and partially walled tablet with the wall at the outer radius; and a capped tablet with the partial wall nearer to the centre.
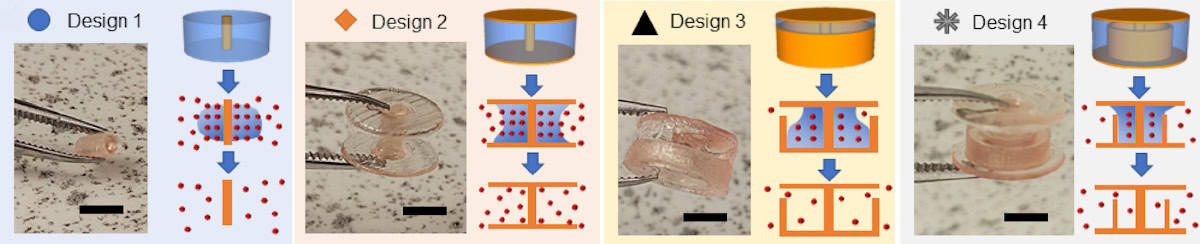
Measuring the release of poly-ACMO from tablets immersed in phosphate-buffered saline revealed that design 1 had the fastest release rate (4.07 mg/min). The insoluble barriers in design 2 reduced the release rate (to 2.17 mg/min), while the outer wall of design 3 reduced it further (to 0.98 mg/min). Design 4 showed an initial release comparable to design 2, then transitioned to a significantly lower release rate (0.70 mg/min) as the poly-ACMO front moved through the inner insoluble wall.
As the poly-ACMO dissolves, the tablets release aspirin with the same profiles: fast release for design 1, slow for designs 2 and 3, and a two-stage release profile for design 4. These findings demonstrate how co-printing soluble and insoluble structures can be used to programme drug release and introduce complexities, such as stepped drug release profiles, that can’t be achieved by traditional manufacturing methods.
The researchers note that the design flexibility of MM-IJ3DP holds potential for incorporating multiple pharmaceuticals in a single tablet, as well as manufacture of different tablet designs in a single production batch of 56 pills.

AI for more intelligent drug discovery
These unique capabilities could advance the field of personalized pharmaceuticals, the researchers say, enabling control of dose (by varying the quantity of drug-containing soluble material), release rate (governed by the surface area) and the time of release (controlled by the spatial distribution of the soluble component).
The team is now working to expand its database of formulations and design pills that maximize the technique’s capabilities. “We are currently investigating the use of AI technology to understand the drug release profiles of different 3D-printed pills,” He tells Physics World. “This approach will offer us a new toolset to help us design pills that meet the desired drug release performance specified by pharmacists for patients.”