Thermoradiotherapy is a cancer treatment in which hyperthermia – heating the tumour to above body temperature – is used to enhance the efficacy of radiotherapy. The amount of this enhancement is expressed as EQDRT, the equivalent radiation dose needed to achieve the same therapeutic effect without heating.
Clinical trials have shown that this approach can substantially improve treatment outcomes in several tumour types, without increasing normal tissue toxicity. Previous studies also demonstrated that both the achieved temperature and the time interval between radiotherapy and hyperthermia impact the clinical outcome.
To understand this process in more detail and help optimize treatments, researchers at Amsterdam UMC have used biological modelling to investigate the impact of maximum temperature and time interval on EQDRT. Describing their findings in the International Journal of Radiation Oncology Biology Physics, they report that both high temperatures and short time intervals are essential to maximize therapeutic enhancement.
Biological model
To perform thermoradiotherapy, clinicians use a radiofrequency or microwave device to apply heat to the tumour once or twice a week, either before or after a radiotherapy session. Tumour temperature is kept below 45°C to prevent heating normal tissue, but sometimes unwanted (and painful) hot spots can occur, which limit the maximum tolerable power level that can be used during a hyperthermia treatment.
First author Petra Kok and colleagues developed software to model the biological effects of radiotherapy plus hyperthermia in terms of equivalent dose distributions. The model, which accounts for DNA-repair inhibition by hyperthermia, as well as direct heat-induced cytotoxicity, enables evaluation of the quality of combined treatment plans using standard dose–volume histograms.
To obtain basic insight into the impact of hyperthermia parameters, the team first calculated the enhancement of a standard 23 × 2 Gy dose distribution by homogeneous temperatures of between 37 and 43 °C, for time intervals between 0 and 4 h.
The model showed that EQDRT increased significantly with both increasing temperature and decreasing time interval. For a 1 h time interval, for example, it predicted an EQDRT increase of 2–15 Gy for temperatures from 39 to 43°C. These findings emphasize the importance of achieving the highest tolerable tumour temperature to optimize clinical outcome.
The impact of time interval was most pronounced at higher temperatures (above 41°C). At a typical hyperthermic temperature of 41.5°C, an EQDRT increase of about 10 Gy was achieved with a 0 h time interval. This decreased to around 4 Gy enhancement with a 4 h interval, indicating that as the time interval increases, a higher temperature is needed to realize the same effect.
Clinical cases
Next, the researchers evaluated realistic treatment scenarios based on inhomogeneous temperature distributions and clinical radiotherapy plans. They calculated the EQDRT for 10 patients with locally advanced cervical cancer. All patients had received 23 × 2 Gy volumetric-modulated arc therapy (VMAT), with hyperthermia applied weekly during the treatment course.
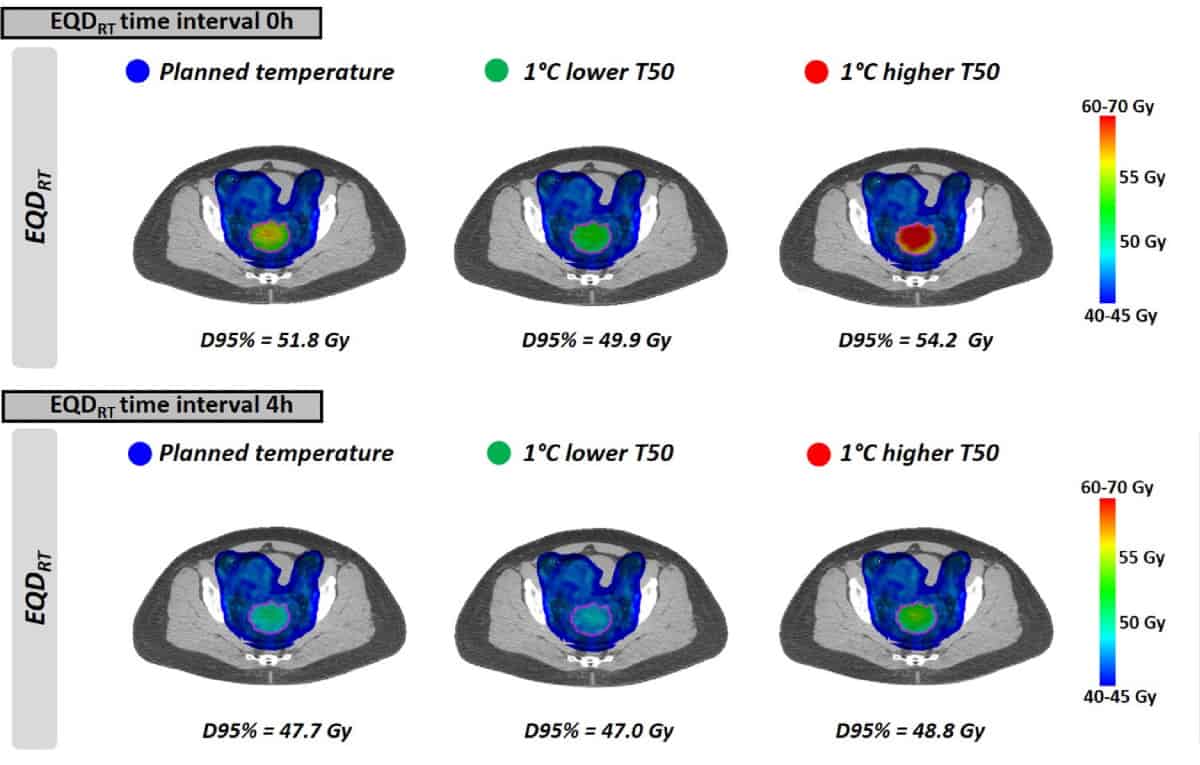
As seen with the uniform temperatures, EQDRT was largest for the smallest time interval. When hyperthermia was applied immediately before or after radiotherapy (0 h time interval), the mean EQDRT to 95% of the volume (D95%) was 51.7 Gy – a gain of 6.3 Gy over radiation alone. Increasing the time interval to 4 h reduced this gain to 2.2 Gy.
The model predicted that most of the dose enhancement is lost within the first hour. For clinical use, therefore, the time between radiotherapy and hyperthermia delivery should be as short as possible – ideally by patients receiving both treatments in the same hospital. The team note that while the order of the two treatments is not clinically relevant, as it takes time to heat up the tumour, applying hyperthermia first could enable significantly shorter time intervals, even close to 0 h.
Finally, the researchers modelled the impact of achieving slightly lower tumour temperatures than planned, due to the occurrence of treatment-limiting hot spots. The effect on EQDRT was most pronounced for a short time interval between radiotherapy and hyperthermia. For a 1°C lower temperature and a 0 h time interval, for example, the mean predicted EQDRT(D95%) decreased by 1.8 Gy (from 51.7 to 49.9 Gy); for a 4 h interval, the decrease was about 0.7 Gy.
Radiotherapy is more effective in warmed-up tumours
In cases where no hot spots appear, it may be possible to increase the output power and reach a higher temperature than planned. Once again, the benefit of achieving a higher temperature was greatest for shorter time intervals, with the exact gain dependent upon the actual temperatures reached.
“Biological modelling provides relevant insight into the relationship between treatment parameters and expected EQDRT,” Kok and colleagues conclude. “Both high temperatures and short time intervals are essential to maximize EQDRT.



