More than 3.6 billion diagnostic imaging exams are performed each year across the globe, with medical radiation use accounting for 98% of the population’s dose from artificial sources. To keep track of this radiation burden, radiology departments employ dose management systems that extract information from X-ray exams to estimate patients’ radiation levels or flag suspicious dose outliers.
The size of a patient will influence their individual organ doses. But for projection radiography (two-dimensional X-ray imaging), dose management systems don’t usually have access to such information. Instead, they use conversion factors based on a reference patient, resulting in less accurate dose calculations.
To address this shortfall, researchers in Belgium have developed a metric to estimate patient size directly from the X-ray images. Importantly, the new metric only uses parameters available in the header of the patient’s DICOM files (which store medical images and related data). They describe the approach in Physics in Medicine & Biology.
“Having a structured, validated methodology for size estimation using DICOM header information paves the way for automated size-specific dosimetry in digital radiography,” explains Hilde Bosmans from the University Hospitals Leuven. “With the widespread adoption of dose management systems that track the patient’s dosimetric records, patent-specific effective organ dose calculations will allow for better data in terms of radiation-induced risks. This is valuable for all patients, and particularly for obese or thinner patients and patients that undergo X-ray exams regularly.”
Metric definition
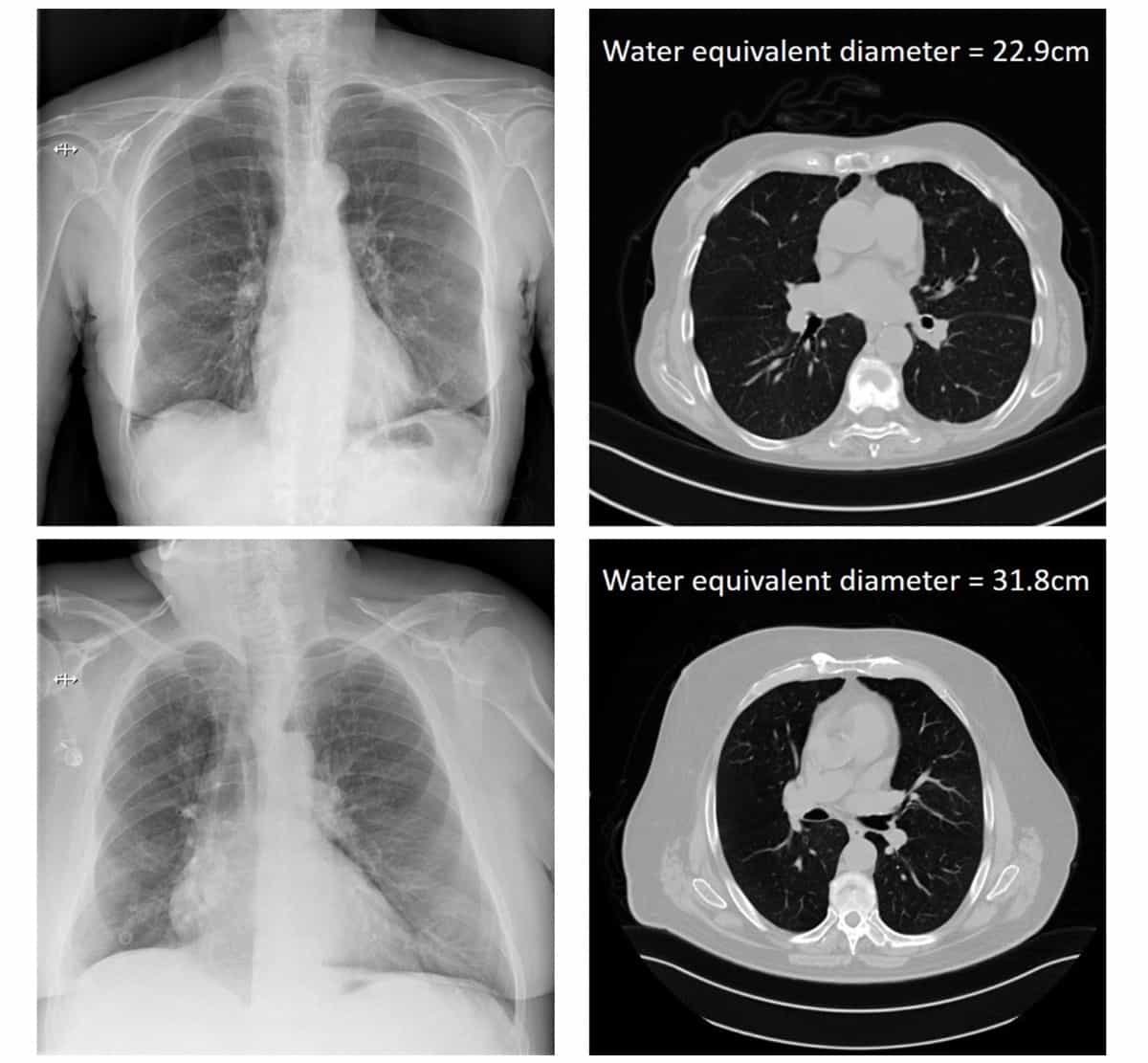
Bosmans and colleagues proposed an attenuation metric related to the dose absorbed in the patient – based on the ratio of incident air kerma to detector air kerma – that correlates with patient size. They defined this metric for both thoracic and abdominal projection radiography, using 137 thoracic and 137 abdominal projection images as input data. These patients also had recent CT exams of the same body part, serving as the gold standard for patient size.

To establish the ground-truth patient size, the researchers used the CT scans to calculate the water equivalent diameter (WED) and water equivalent thickness (WET) of all patients. They then plotted these ground-truth WED and WET values versus the natural log of the attenuation metric, for both thoracic and abdominal scans. This generated four correlation curves that could then be applied to estimate patient size based solely on DICOM information from the projection radiographs.
The team note that some of the DICOM fields required for this approach (exposure index, kerma–area product, exposed area and source–detector distance) are optional. “They are, however, usually available in the DICOM header or could be made available by the system vendor,” says Bosmans. “In several countries, it is even mandatory to have these data displayed. After all, they are important indices to monitor the practice and the equipment, and they can unravel problems or occasional malpractice.”
The researchers validated the technique’s ability to estimate patient size using four different radiography systems. For all devices, they examined X-ray exams from 50 new patients and used the correlation curves to estimate WED and WET values based on the DICOM information.
Three of the systems (Carestream’s DRX Evolution, Siemens’ Axiom Luminos dRF and Canon’s CXDI-11) included a standardized exposure index, in which the exposure index is linearly proportional to the detector air kerma (rather than being vendor-specific), thereby enabling consistent performance evaluation across devices and departments.
For thoracic exams on these systems, the differences between estimated and ground-truth WED were all within ±15%, with absolute differences of 4% on average. Estimated WET values had absolute differences of 8%, 7% and 7%, for DRX Evolution, Axiom Luminos dRF and CXDI-11, respectively. In the two systems used to perform abdominal scans, the average absolute differences between estimated and ground-truth values were 4% and 6% for WED, and 6% and 8% for WET.
The researchers also examined a system without standardized exposure index: the Triathlon DR from Oldelft. For thoracic exams, the technique underestimated WED and WET, with average differences from the ground truth of –36% and –57%, respectively. For abdominal scans, the algorithm gave similar results to the other systems, with deviations of 3% for WED and 5% for WET.
Improved accuracy
The researchers suggest that the new metric could enable individualized risk assessments with better accuracy than using a generic conversion factor. They emphasize that their method is in principle applicable to all devices that acquire X-ray projections. Including patient size in a dose management platform would improve the dosimetric data and improve dose outlier management by reducing false positives from overweight patients and false negatives due to underweight patients.
Web-based tool estimates foetal radiation dose from CT scans
Bosmans notes that this development is part of a larger project to create, along with personalized CT dose estimates, automated personalized dosimetry for 2D projection imaging. The research is performed in collaboration with the medical software company Qaelum and supported by a Flemish VLAIO grant. The intention is to implement the automated size estimation metric into Qaelum’s dose and quality monitoring software.
“Ultimately, hospitals and patients will benefit from a total solution that will automatically evaluate the quality of the exam at the one side and provide advanced effective and organ dose estimations in X-ray imaging at the other,” she tells Physics World.



