The recent introduction of MRI guidance into the radiotherapy treatment chain brings benefits such as high soft-tissue contrast, zero-dose set-up verification and the ability to perform adaptive replanning. In a high-field MR-linac, however, the strong magnetic field causes hot and cold dose spots at interfaces between high- and low-density regions – a phenomenon known as the electron return effect (ERE).
The ERE causes secondary electrons entering a low-density region to be re-directed back toward the high-density region, creating increased dose deposition in the denser tissue. This creates significant dosimetric challenges – particularly in the thoracic region, which contains interfaces between lung, airways, soft tissue and bone – and requires a dosimetry system that can measure steep dose gradients in three dimensions.
To address this need, a team from the MD Anderson Cancer Center has developed a low-density, lung tissue-equivalent gel dosimeter and used it to measure the ERE at a lung–soft tissue interface in an MR-linac (Phys. Med. Biol. 10.1088/1361-6560/ab4321).
The team investigated two Fricke (ferrous sulphate-based) gels: FXG and FOX. “We chose Fricke gels because they’re easily read out with MRI. This is particularly advantageous for our MR-linac set-up as we can irradiate and image the dosimeter before and after irradiation without having to reposition it,” explains first author Brigid McDonald. “Other gels are visible on MRI, but we’ve found the Fricke gels to be the most straightforward to manufacture in the lab. They’re also extensively characterized in the literature, so we have confidence in their linear dose response behaviour.”
As Fricke gels are optimized to represent soft tissue, McDonald and colleagues added various sized polystyrene beads to create low-density gels that mimic lung tissue. All of the resulting gels had CT numbers of around -600 HU, which is slightly higher than typical lung tissue (-700 to -800 HU) but suitable for use in dosimetry.
Adding beads decreased the MRI signal intensity compared with the standard gel. The researchers also observed that all FXG gels exhibited higher signals than their corresponding FOX gels and that image uniformity generally improved with smaller bead sizes. Based on these results, they selected the optimal lung-equivalent gel as FXG with small (less than 1 mm diameter) polystyrene beads. Dose–response curves for both the optimized gel and conventional FXG showed linear changes with dose up to 30 Gy.
Measuring the ERE
The researchers used the optimized gel formulation to create two identical phantoms containing an inner cylinder of low-density gel surrounded by an outer cylinder of conventional FXG, to simulate a lung–soft-tissue interface. They irradiated one phantom in the Elekta Unity MR-linac and the other in a conventional linac, using the MR-linac to image the two phantoms before and after irradiation.
Dose maps generated using the measured dose–response relationships for the two gels showed that the dose decreased as the beam penetrated into the phantom, with a region of lower dose inside the low-density inner cylinder.
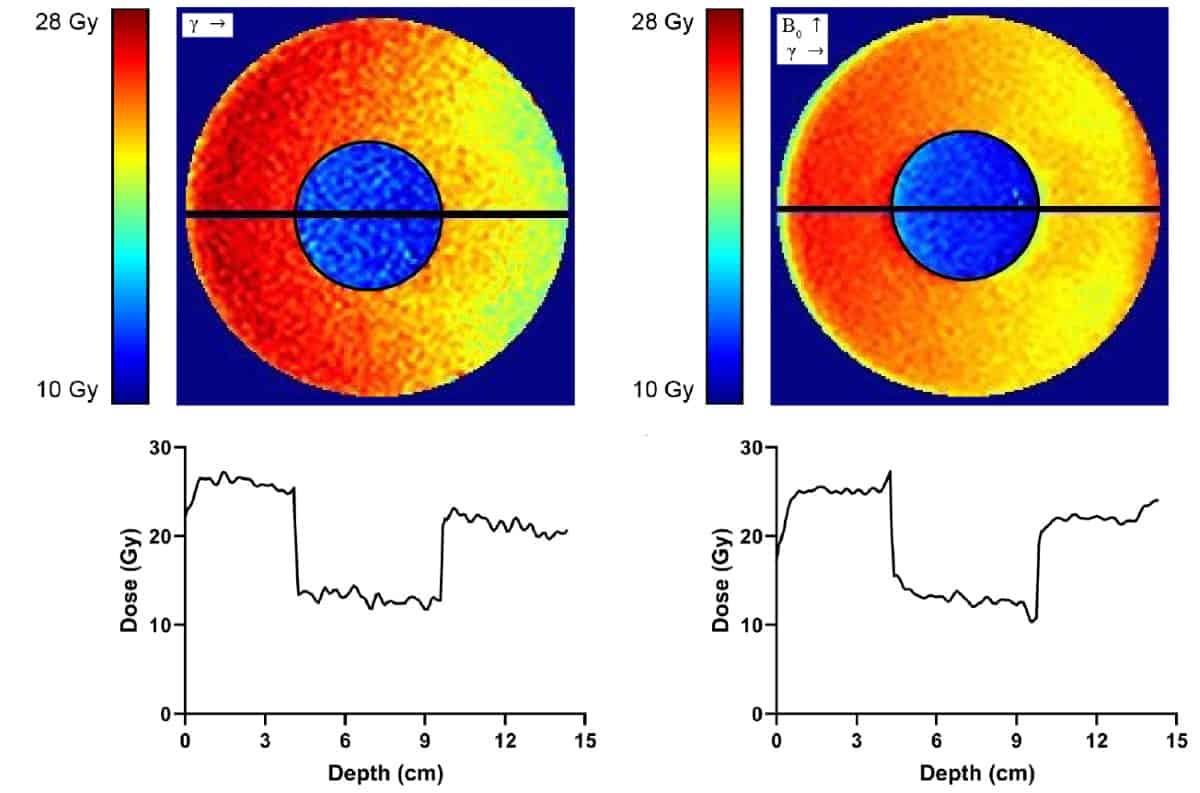
The MR-linac-irradiated phantom exhibited a region of enhanced (by 8.7%) dose in the conventional gel just before the beam entered the low-density cylinder, plus a region of reduced (by 17.7%) dose in the low-density gel just before the beam re-entered the conventional gel. The researchers also saw dose enhancement as the beam exited the phantom into air. These hot and cold spots did not appear in the dose map of the linac-irradiated phantom, indicating that they arise from the ERE caused by the magnetic field.
The researchers suggest that the linear dose–response curve seen for the low-density gel shows that it can be used reliably for MR-linac dosimetry up to 30 Gy, though its high-dose behaviour still needs to be determined.
“The focus of this study was to optimize the low-density gel formulation and characterize the uncertainty in the measured dose,” McDonald tells Physics World. “The next steps would be to take CT scans of these phantoms to load into the treatment planning system, calculate the predicted dose distribution, deliver the plan to the phantom and then compare the expected dose to the MRI-measured dose.”

3D printing enables gel dosimetry in complex phantoms
She explains that this method may be a bit more laborious than using a standard QA device such as a diode array, but emphasizes that standard QA devices can’t measure the dosimetric impact of the ERE at density boundaries.
The team plan to start these validation tests using simple phantom geometries, then move on to anthropomorphic phantoms for applications in clinical MR-linac treatments. “One of the cool things about gel dosimeters is that they can be moulded to fit a container of any shape,” explains McDonald. “So we can use our low-density gel to generate anthropomorphic phantoms of the thoracic region, to approximate the radiation dose delivered to a patient.”