The ability to accurately track the movement of cancer cells as they metastasize to other locations in the body could allow oncologists to better target therapies and start treatments earlier. To achieve this, researchers at Stanford University School of Medicine have developed CellGPS, an extremely sensitive and accurate method of single cell tracking using positron emission tomography (PET). They describe the technique and its feasibility to track single cell migration in laboratory mice in Nature Biomedical Engineering.
Applications for single cancer cell tracking using CellGPS include modelling and investigating the earliest stages of metastatic migration, including the circulation of tumour cell clusters in the blood, and the role of haemodynamic forces, cell adhesion, blood flow and biomechanical interactions. CellGPS could also be used to non-invasively measure the routes and kinetics of immune-cell mobilization in response to injury.
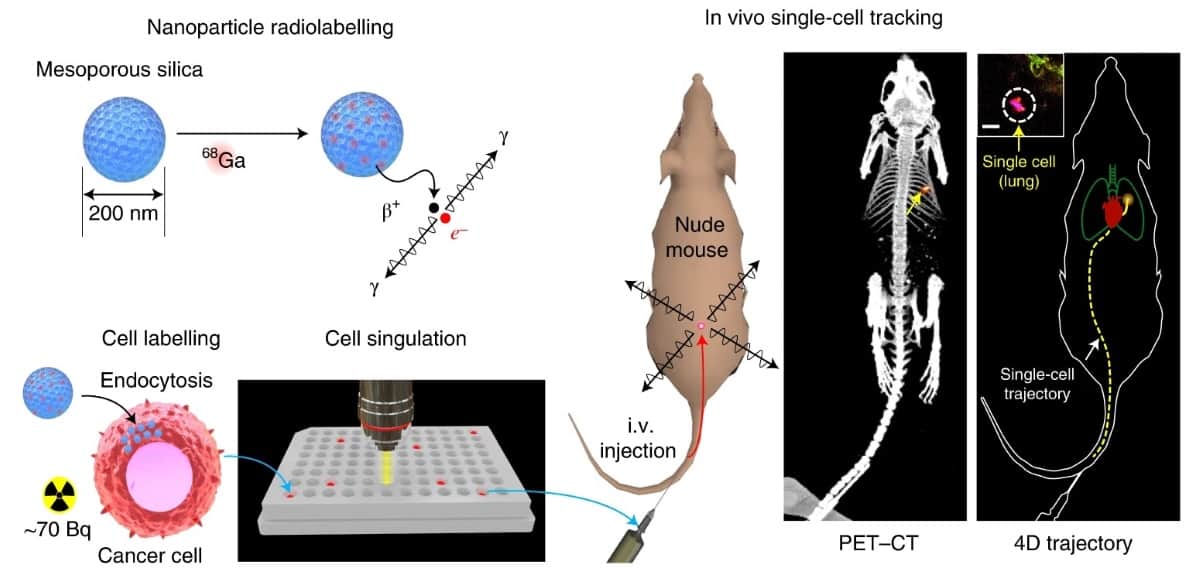
The CellGPS technique uses mesoporous silica nanoparticles (MSNs) to efficiently transfer radionuclides into cells for real-time cell tracking. The researchers labelled the nanoparticles with gallium 68 (68Ga), a radiometal with a 67-min half-life that’s commonly used for PET imaging of neuroendocrine tumours and metastatic prostate cancer. To enhance cell loading, they coated the nanoparticles with a lipid bilayer. MSNs with a diameter of 200 nm and a pore size of 4 nm carried 68Ga into live MDA-MB-231 breast cancer cells (a common model of breast cancer metastasis to the lungs), achieving a radioactivity level of nearly 100 Bq per cell.
The researchers initially assessed the metabolic activity of the cells after radiolabelling, noting that there was a small (5–15%) decrease in overall metabolic activity within 24 hours. They verified that DNA damage in cells, although detectable, did not result in significant biological differences in apoptosis, proliferation and migration.
After determining the optimal settings for acquiring PET images and data, the researchers injected approximately 100 breast cancer cells labelled with 68Ga-MSNs intravenously into mice. They used PET to confirm that the cancer cells arrested in the lungs. When they subsequently reduced the number of injected cells to just one, PET-CT showed a single focus of radiotracer accumulation in their lungs, appearing as a hot spot of approximately 1.7 mm diameter.
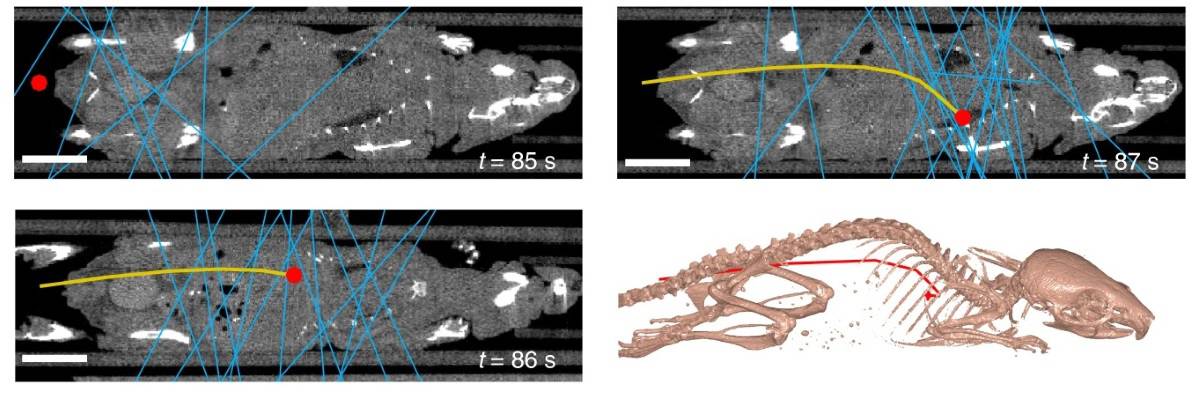
The researchers also used PET data to dynamically track the position of a moving cell in vivo. Using the list-mode data from the scanner, they computed the 3D position of a single cell injected into a tail vein through a butterfly catheter. The cell, traveling at a velocity of up to 50 mm/s, was tracked to the lungs within 3 s of intravenous injection. Its location matched the focal signal seen on conventional PET-CT imaging.
To demonstrate that single cells can be imaged beyond the 67 min half-life of 68Ga, the researchers also tested the CellGPS workflow using zirconium-89 (89Zr, a positron-emitting radionuclide with a half-life of 78 hr). They intravenously injected single cells labelled with 89Zr-MSNs into two mice and imaged them over multiple days using PET. A single focus of uptake could be detected in the lungs for up to 48 hr and remained stable.

Principal investigator Guillem Pratx and colleagues state that the two most important findings of their research are that PET is sufficiently sensitive to image static single cells labelled with more than 20 Bq per cell of 68Ga, and that single migrating cells can be tracked dynamically directly from list-mode PET data.
“The CellGPS methodology could be extended to enable tracking of multiple cells simultaneously in the same individual,” they explain. “In the case of cellular therapies in which millions of cells are injected, it will be possible to label and track a small subset of cells using the CellGPS methodology.”
“We plan to push the technology to enable tracking of multiple single cells at the same time in the same individual by multiplexing the PET signals,” Pratx tells Physics World. “We also plan to investigate new applications where cell migration is key, primarily in the area of immune cell tracking for cancer immunotherapy applications, stem cell regenerative medicine, and more advanced models of cancer metastasis.”