The growing clinical application of stereotactic radiosurgery (SRS) for the treatment of metastatic tumours in the brain presents a significant dosimetric and quality assurance (QA) challenge for medical physicists and their clinical colleagues. Put simply, the precision targeting inherent to SRS necessitates all manner of patient, machine and process-level checks to verify that conformal, high-dose radiation is delivered to the patient as intended – usually in one or a few fractions – while minimizing damage to surrounding healthy tissue and organs.
With this in mind, Modus QA, a Canadian supplier of QA products and services to radiation oncology clinics, is stepping up the commercial roll-out of its ClearView 3D Dosimeter, a non-diffusing, radiochromic hydrogel dosimeter designed specifically to support advanced radiotherapy techniques like SRS. Working in tandem with the vendor’s VISTA optical CT scanner, the dosimeter helps users to confirm that planned treatments are delivered accurately, visualizing the intricate detail of complex dose distributions for multiple-lesion dosimetry.

“Gel dosimetry can be used to measure any 3D dose distribution,” explains John Miller, founder and co-owner of Modus QA, “though the most important application we see is for SRS treatment of metastatic tumours in the brain – a truly complex problem with multifoci targets distributed in 3D space.” Fundamentally, ClearView is a tool that enables medical physicists to meaningfully compare 3D dose distributions – measured versus calculated – and get them to a pass or fail ahead of SRS treatment. “It’s a necessary and sufficient test,” adds Miller.
Joined-up thinking
If that’s the back-story, what of the specifics? The ClearView 3D Dosimeter itself comprises an optically clear, low-scattering and colourless hydrogel matrix suffused with a radiochromic indicator dye. The dye turns purple after irradiation, with the change in optical attenuation throughout the gel directly proportional to the absorbed radiation dose.
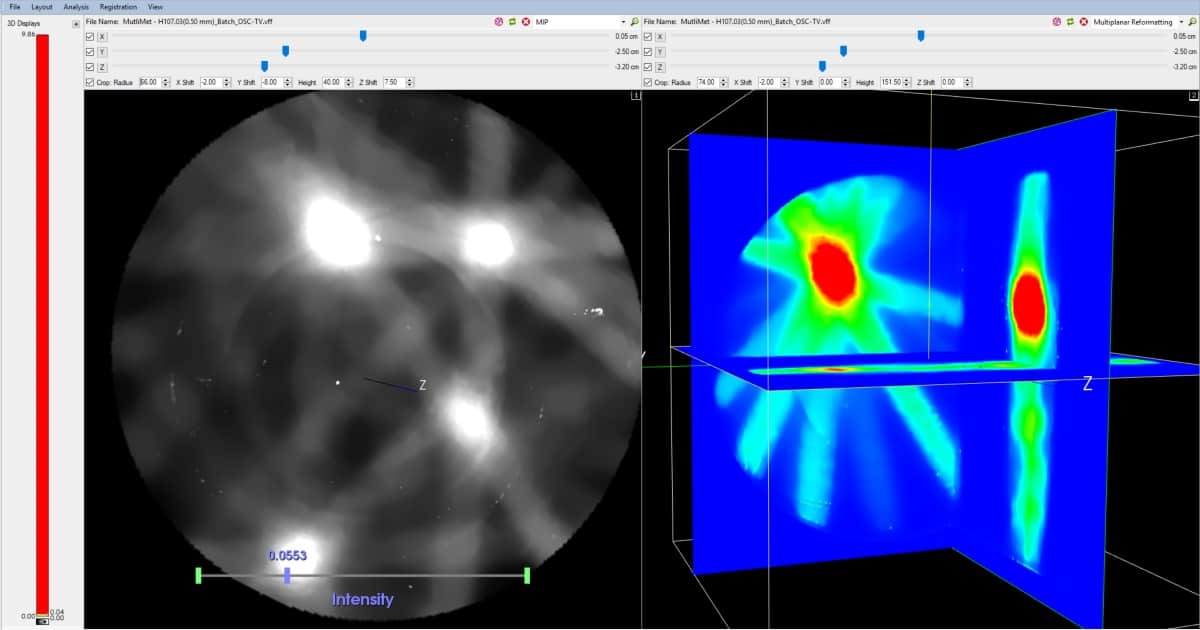
Spatially, while the radiochromic dose response of the ClearView gel is accurate at the submicron scale, the resolution of the dose image is ultimately determined by the spatial accuracy of the optical CT scanner used for readout. In this case, the latest iteration of the Modus optical CT scanner (VISTA 16) is capable of imaging 0.25 mm isotropic voxel sizes, though in practice the more commonly used spatial resolution of 0.5 mm will be down-sampled to match the treatment plan.
Also worth noting is the joined-up approach that Modus has taken to ClearView and VISTA product development. VISTA, for example, is built with a convergent green light source (530 ± 10 nm) to minimize the scatter associated with cone-beam optical CT, while ClearView has been optimized for use with VISTA by increasing the clarity and reducing the scatter of the hydrogel. “The goal is an integrated 3D dosimetry system,” Miller explains. “The combination of the two products ultimately gives users more accurate measurements of dose and dose distribution.”
Into the workflow
When it comes to deploying ClearView and VISTA into the SRS QA workflow, Miller reckons an experienced user will need about 60 minutes to measure a dose distribution and compare it to the calculated dose distribution. The stepwise process includes acquisition of an optical CT scan of the gel before irradiation (the reference scan); irradiation of the gel with the planned treatment dose; a 45-minute wait for the gel chemistry to develop; followed by another optical CT scan (the data scan). Each scan takes less than five minutes, while the CT reconstruction is automatic.
“It’s the comparisons at this point that are crucial,” says Miller. Users can do a gamma distribution to see a gamma map in 3D; also a conformity index between planned isodose surfaces and measured isodose surfaces. In a multimet treatment, meanwhile, users are able to look at the centre of mass of each target and compare with the plan to ensure the centre of each target is hit with the centre of each dose distribution. “You can then look at contours around that to make sure the shape of the dose distribution is correct,” he adds.
The ClearView roadmap
Looking ahead, the priorities for 2020 are already nailed down for the Modus product development team. With the ClearView roll-out under way to new clinical customers, the emphasis now shifts to commercial release – slated for the autumn – of the work-in-progress VistaACE analysis software. “The software will effectively complete the product launch,” notes Miller, “enabling medical physics teams to use ClearView, VISTA and VistaACE on site as an integrated solution for 3D gel dosimetry.”
Beyond that, intriguing possibilities are coming into view on the ClearView development roadmap. A case in point is a concept called 3D dosimetry as a service (3DDaaS). “There’s no timeline for the launch of 3DDaaS as yet,” Miller admits, “but the idea is that this will be a remote accreditation service for QA of clinical procedures – for example, to support the commissioning of new radiotherapy equipment or new treatment techniques in the clinic.”
Right now, Modus is focused on accelerating clinical uptake and validation of ClearView beyond its current “early-adopter” institutions, which include the London Regional Cancer Program in Ontario and the University of Michigan. A key opportunity to engage with end-users directly will come in June at the International Conference on 3D and Advanced Dosimetry in Quebec City.
“Watch this space,” Miller concludes. “We’ll be there with at least the work-in-progress VistaACE.”
Using the ClearView 3D Dosimeter
Selling the benefits of gel dosimetry to the medical physics community is all about openness, claims John Miller of Modus QA. “Transparency is key,” he explains. “We’ve tested ClearView rigorously and are being upfront and open with all our specifications – acknowledging that radiochromic gels have their limitations with respect to linearity, range and energy and dose-rate effects.”
Benefits of the Modus QA ClearView 3D Dosimeter include:
- Stability: chemistry is stable for more than 60 days prior to irradiation under recommended storage conditions. The signal is geometrically stable any time after irradiation (though for best dosimetric results, Modus recommends optical scanning between 45 min and 24 hr after irradiation).
- Linearity: dose response is linear within the range 0 to 80 Gy, making ClearView suitable for SRS QA.
- Spatial resolution: the gel is accurate to the submicron level.
- Material properties: near-tissue equivalence provides a patient-like testing environment, while low-scatter substrate is ideal for optical cone-beam CT scanning.
A full list of ClearView specifications is available here.




